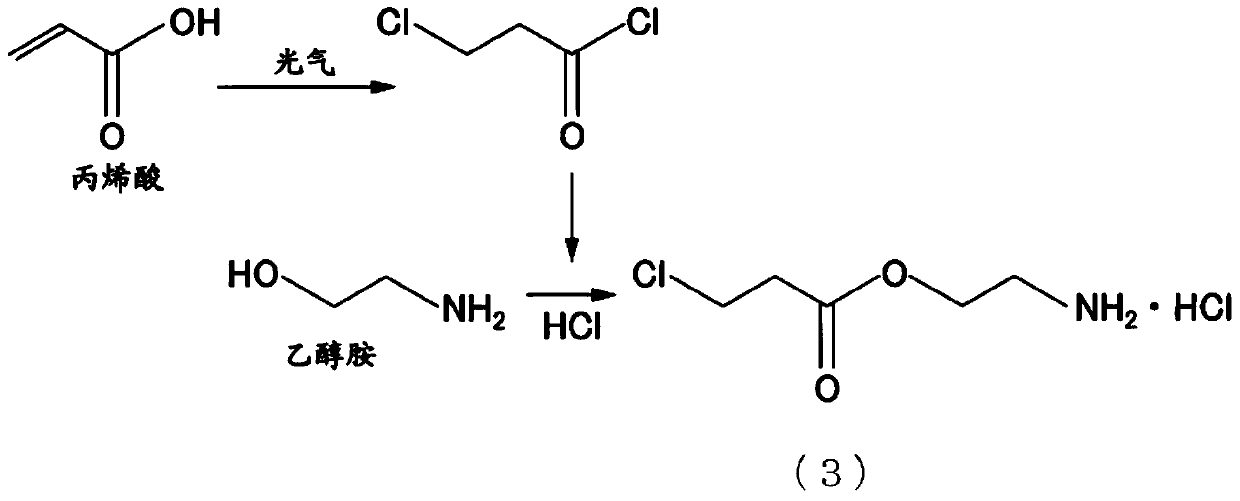
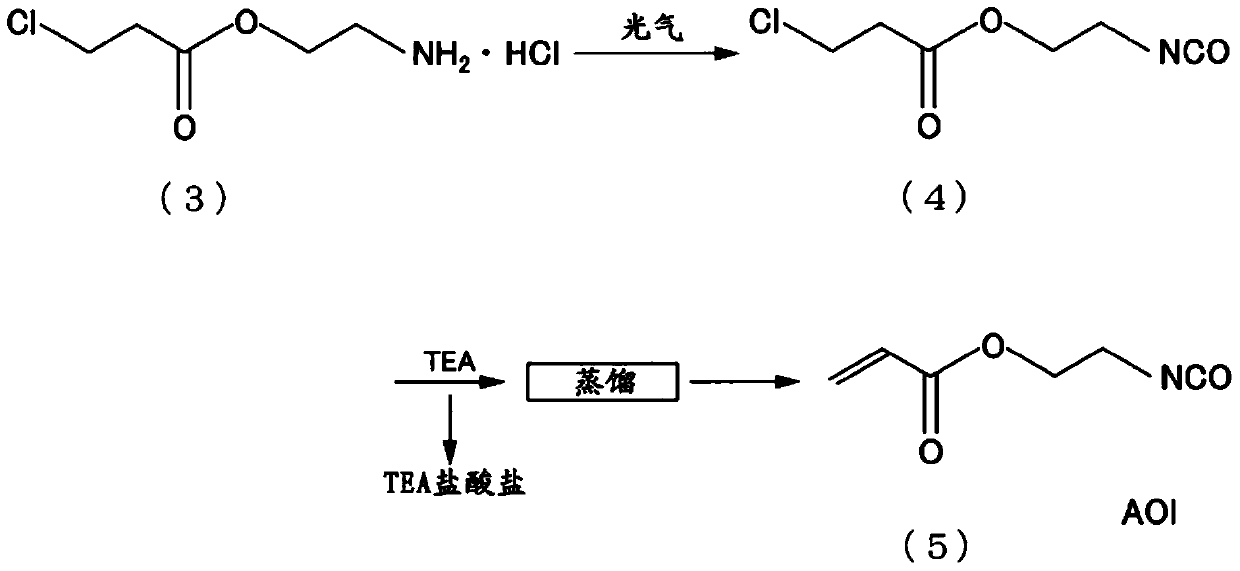
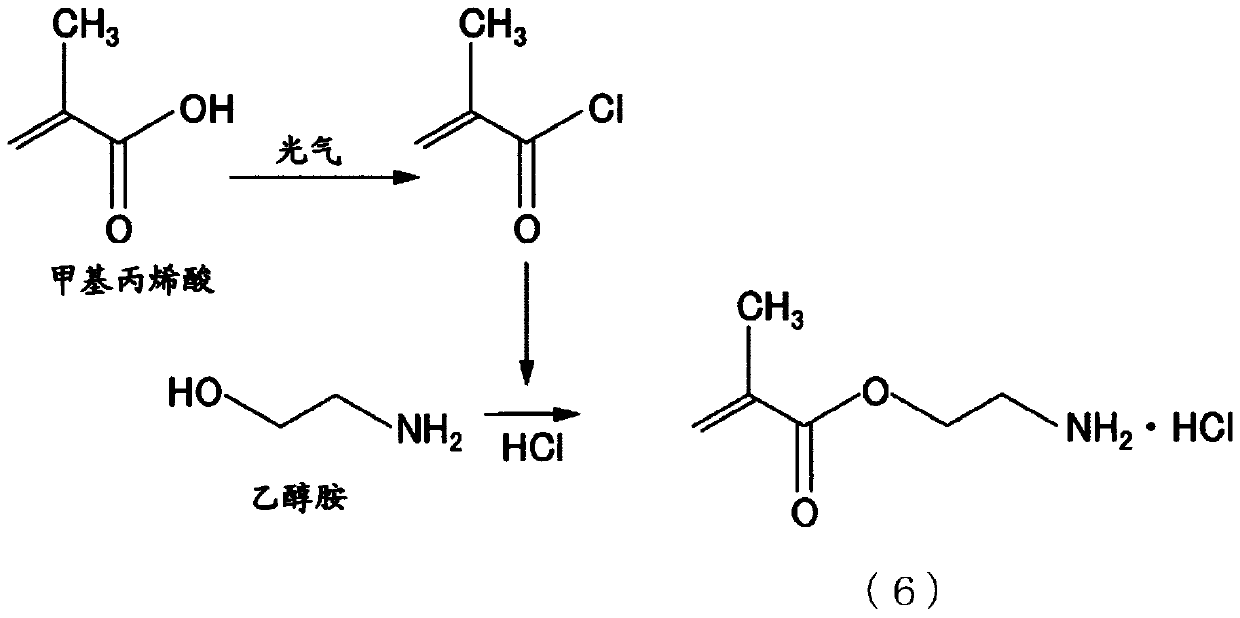
Composition, method for producing composition, unsaturated compound and method for producing unsaturated compound
A manufacturing method and composition technology, applied in the preparation of organic compounds, preparation of urea derivatives, chemical instruments and methods, etc., can solve the problems of low solubility, avoid sharp rise in viscosity, prevent coloring, and excellent stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5、 comparative example 1
[0197] [Examples 1 to 5, Comparative Example 1: MOI storage test and synthesis of unsaturated carbamate compound]
[0198] In any one of MOI 1 to 5 shown in Table 2, 2,6-di-tert-butyl-4-methylphenol (BHT) was added as a polymerization inhibitor in the amount shown in Table 3. Assuming It stored for 14 days at 45 degreeC which is a summer storage condition, and evaluated the appearance by the method shown below. The results are shown in Table 3.
[0199] "Appearance Evaluation"
[0200] ○: No change.
[0201] ×: Polymerization (gelation) occurred.
[0202] Add any one of 77.5 g of MOI1 to 5 and 165 g of polyethylene glycol (number-average molecular weight 660) that have undergone the above-mentioned storage treatment into a four-necked flask with a capacity of 500 mL equipped with a stirrer, a reflux cooling tube, and a thermometer, The temperature was maintained at 80° C. for 5 hours to synthesize an unsaturated carbamate compound.
[0203] The viscosity (Pa·sec) at 25° C...
Embodiment 6~10、 comparative example 2
[0211] [Examples 6-10, Comparative Example 2: AOI storage test and synthesis of unsaturated urethane compound]
[0212] The polymerization inhibitor (BHT) similar to Example 1 was added to any of AOI1-5 shown in Table 1 by the addition amount shown in Table 4, and it stored and evaluated similarly to Example 1. The results are shown in Table 4.
[0213] Add 70.5 g of any one of AOI1 to 5 that has undergone the above-mentioned storage treatment, and 165 g of polyethylene glycol (number average molecular weight 660) into a four-necked flask with a capacity of 500 mL equipped with a stirrer, a reflux cooling tube, and a thermometer, The temperature was maintained at 80° C. for 5 hours to react to synthesize an unsaturated urethane compound, which was stored at room temperature for 1 week, and the appearance was evaluated by the method shown below. The results are shown in Table 4.
[0214] "Appearance Evaluation"
[0215] ○: No change.
[0216] ×: Polymerization occurred (inc...
Embodiment 11、 comparative example 3
[0224] [Example 11, Comparative Example 3: Synthesis of Unsaturated Urethane Compound]
[0225] Add 273g of AOI4 or AOI5 shown in Table 1, and 72.4g of pentaerythritol to a four-necked flask with a capacity of 500mL equipped with a stirrer, a reflux cooling tube, and a thermometer, and add 2,6-di-tert-butyl- 4-Methylphenol (BHT) was made into 50ppm, and the temperature was maintained at 80° C. for 5 hours to react to synthesize an unsaturated carbamate compound, which was stored at room temperature for 1 week. The same operation was performed as in Example 6, and the appearance was checked. Evaluation.
[0226] As a result, problems such as coloring and gelation were not observed in the unsaturated urethane compound of Example 11 using AOI4 having a triethylamine (TEA) hydrochloride content of 0.01 to 10 ppm.
[0227] On the other hand, the unsaturated urethane compound of Comparative Example 3 using AOI5 in which the content of triethylamine (TEA) hydrochloride was higher th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com