Polypeptide and nucleic acid coupling compound used for targeted therapy
A technology for coupling compounds and nucleic acids, applied in the field of biomedicine, can solve problems such as safety issues, technical difficulties, and complicated processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
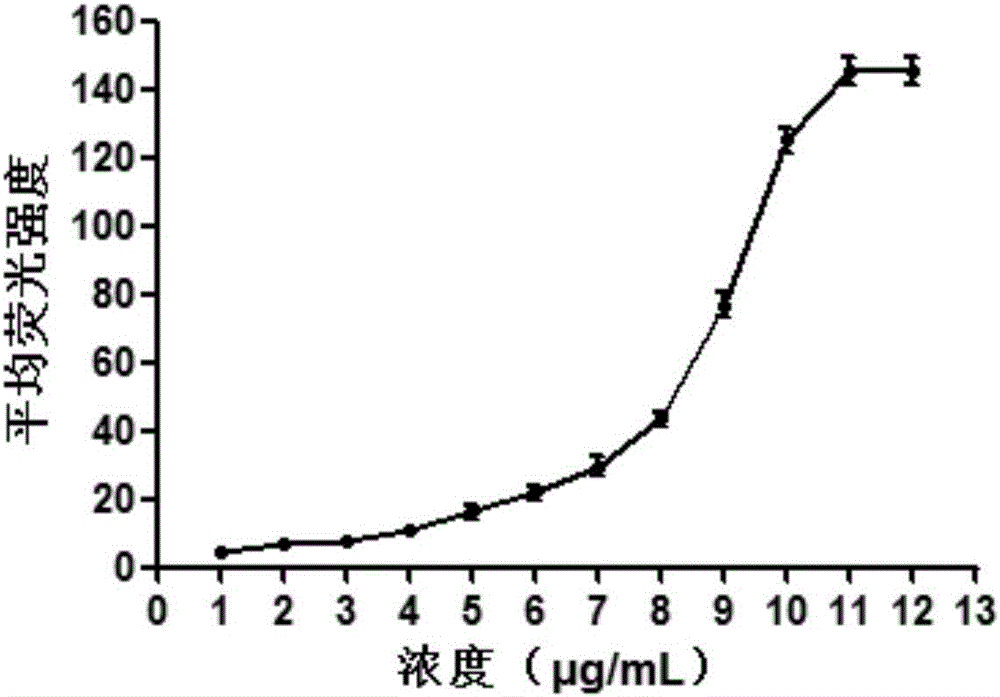
[0030] Example 1 Azide-labeled GnRH polypeptide has targeting and specificity
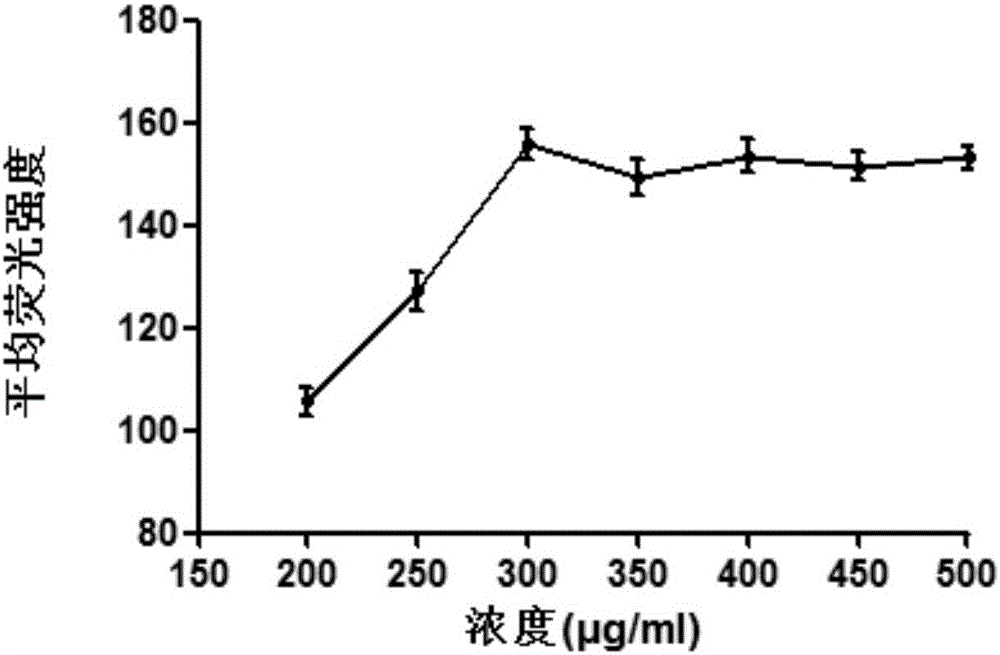
[0031] Hela cells with high expression of GnRHR were selected as experimental cells to verify the binding of [D-Lys6]GnRH to different concentration gradients of Hela cells. Hela cells were seeded in 6-well plates, and when the confluence of the cells reached 70% to 80%, different concentrations of fluorescently labeled [D-Lys 6 (FITC)] GnRH (purchased from Beijing Zhongke Yaguang Biotechnology Co., Ltd.), after 6 hours, the cells were digested with trypsin, and the supernatant was discarded by centrifugation; resuspended in PBS, and the supernatant was discarded by centrifugation (repeat three times), Removal of unbound [D-Lys 6 (FITC)] GnRH, finally add 0.5mL PBS, resuspend, and detect by flow cytometry (BD company), flow cytometry results are analyzed using FlowJo 7.6.1 software, the results are shown in figure 1 . From figure 1 Visible, [D-Lys 6 (FITC)] GnRH binds to receptors on cells in ...
Embodiment 2
[0034] Example 2 [D-Lys 6 ] Synthesis, isolation, purification and verification of GnRH-siRNA
[0035] This embodiment synthesizes [D-Lys by click reaction 6 ] GnRH-siRNA, and then the product was isolated and purified by UPLC, and the product was verified by UPLC-MS.
[0036] Experimental materials and reagents: [D-Lys 6 (azidopentanonic acid)] GnRH (synthesized by Beijing Zhongke Yaguang Biotechnology Co., Ltd.), PLK1 siRNA modified by butynyl at the 5' end of the sense strand (sense strand: 5'-CH≡C-CH 2 -CH 2 (mU)GAAGAAGA(mU)(mC)A(mC)(mC)(mC)(mU)(mC)(mC)(mU)(mU)A dTdT-3'("m"in the sequence represents the 2' position Methoxy modification is 2'-OMe, the same below, see SEQ ID No: 2), antisense strand: 5'-UAAGGAGGGUGAUCUUCUUCA dTdT-3' (SEQ ID No: 3), purchased from Guangzhou Ruibo Biotechnology Co., Ltd. ), Dimethylsulfoxide (DMSO), CuSO 4 ·5H 2 O, tert-butanol (t-BuOH), TBTA (tris(benzyltriazolylmethyl)amine), UPLC, ESI-Orbitrap MS.
[0037] Chromatographic column: Ju...
Embodiment 3
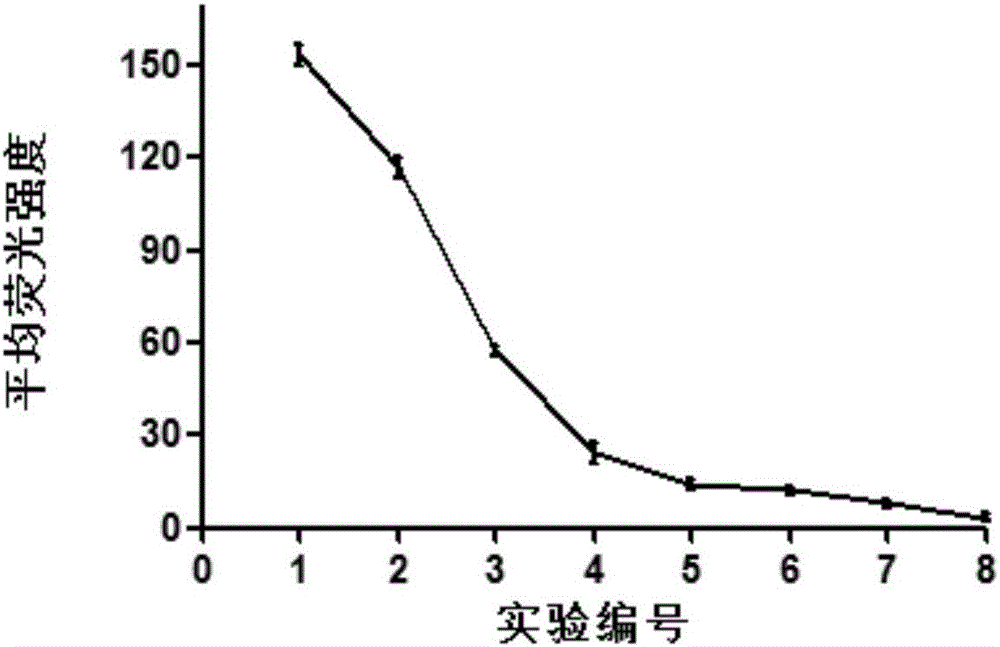
[0040] Example 3 Validation of [D-Lys6]GnRH-siRNA activity after CLICK reaction
[0041]In order to verify the biological activity of the product [D-Lys6]GnRH-siRNA after the click reaction, we detected the expression level of plk1 by qPCR after transfecting [D-Lys6]GnRH-siRNA into Hela cells. Hela cells were seeded into 24-well plates one day before transfection, and the number of cells per well was 5×10 4 , The cell density should reach 30%-50% during transfection. During transfection, the cells were divided into four groups on average, which were blank group (Blank), negative control group (NC), PLK1 group (P+) and click product group (Click+). The blank group did not undergo any treatment, and the negative control group was transfected with lipofectamine 2000 (abbreviated as lip2000 hereinafter) with a stable irrelevant sequence siRNA (sense strand 5'-UUCUCCGAACGUGUCACGUdTdT-3' (SEQ ID No: 4); antisense strand: 5 '-ACGUGACACGUUCGGAGAAdTdT-3' (SEQ ID No: 5)), P+ group was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com