Method for preparing polymerizable photoinitiators
A technology of polymerization photoinitiator and polymerization inhibitor, which is applied in the field of light curing, can solve the problems of high corrosion, complicated method, high toxicity, etc., and achieve the effect of lowering melting point, increasing compatibility and preventing yellowing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
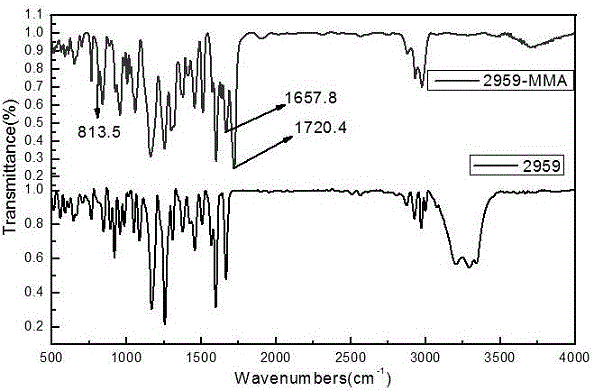
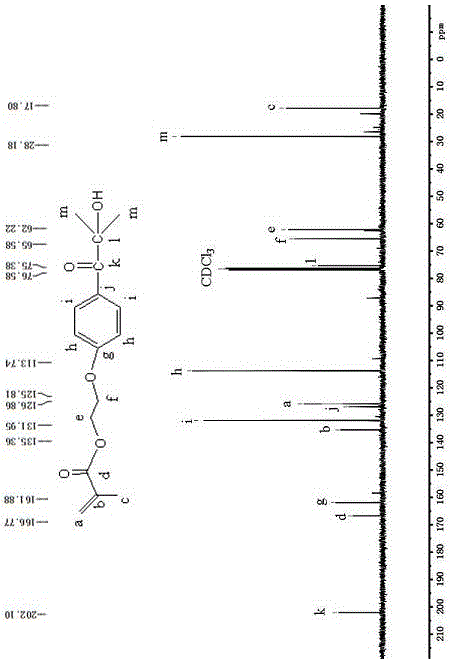
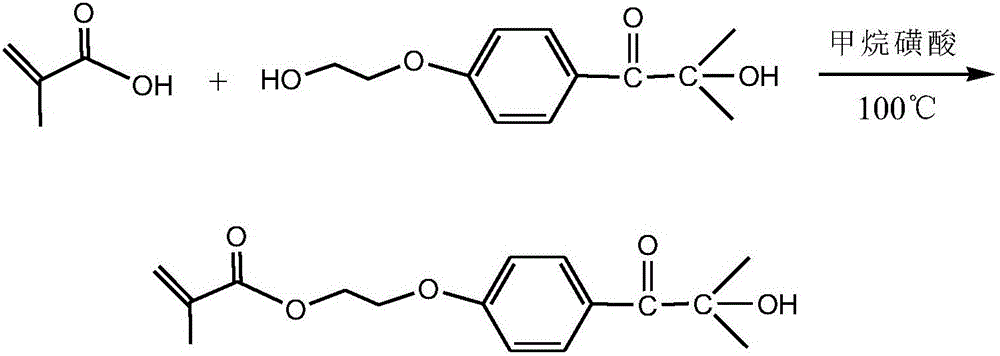
[0020] Take a dried 250mL four-neck flask, respectively install a thermometer, a water separator, a condenser, and flush with nitrogen protection, and add 0.02mol (about 1.72g) of methacrylic acid and 0.01g of p-hydroxybenzoic acid to the flask, respectively. Mix ether and 50mL toluene evenly, add 0.02mol (about 4.48g) 2959, stir to dissolve it completely, add 0.04g catalyst methanesulfonic acid, adjust the reaction temperature to 100°C, and react for 4h. After the reaction, use a rotary evaporator to remove the toluene to obtain a crude product, dissolve the crude product in dichloromethane, and wash it with deionized water and saturated sodium bicarbonate solution several times, dry the washed organic phase with anhydrous magnesium sulfate, and remove it by filtration. Magnesium sulfate, the collected organic phase is further purified through column chromatography, wherein the developer in the silica gel column is a mixed solvent of sherwood oil and ethyl acetate, and its rat...
Embodiment 2
[0022] Take a dried 250mL four-neck flask, respectively install a thermometer, a water separator, a condenser, and flush with nitrogen protection, and add 0.02mol (about 1.72g) of methacrylic acid and 0.01g of p-hydroxybenzoic acid to the flask, respectively. Ether and 50ml of dichloromethane, mix well, add 0.02mol (about 4.48g) 2959, stir to dissolve it completely, add 0.04g catalyst methanesulfonic acid, adjust the reaction temperature to 100°C, and react for 4h. After the reaction, use a rotary evaporator to remove the toluene to obtain a crude product, dissolve the crude product in dichloromethane, and wash it with deionized water and saturated sodium bicarbonate solution several times, dry the washed organic phase with anhydrous magnesium sulfate, and remove it by filtration. Magnesium sulfate, the collected organic phase is further purified through column chromatography, wherein the developer in the silica gel column is a mixed solvent of sherwood oil and ethyl acetate, a...
Embodiment 3
[0024] Take a dried 250mL four-neck flask, respectively install a thermometer, a water separator, a condenser, and flush with nitrogen protection, and add 0.02mol (about 1.72g) of methacrylic acid and 0.01g of p-hydroxybenzoic acid to the flask, respectively. Ether and 50ml of dimethylformamide, mix well, add 0.02mol (about 4.48g) 2959, stir to dissolve it completely, add 0.04g catalyst methanesulfonic acid, adjust the reaction temperature to 100°C, and react for 4h. After the reaction, use a rotary evaporator to remove the toluene to obtain a crude product, dissolve the crude product in dichloromethane, and wash it with deionized water and saturated sodium bicarbonate solution several times, dry the washed organic phase with anhydrous magnesium sulfate, and remove it by filtration. Magnesium sulfate, the collected organic phase is further purified through column chromatography, wherein the developer in the silica gel column is a mixed solvent of sherwood oil and ethyl acetate,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com