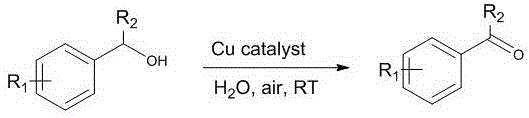Method for preparing aldehyde or ketone by alcohol selective oxidation under catalysis of copper complex
A copper complex and selective technology, which is applied in the direction of oxidation preparation of carbonyl compounds, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problems of poor solvents and achieve convenient preparation and low price , a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the synthesis of copper complex
[0022] Dissolve N-methylimidazole (82 mg, 0.001 mol), 2,2,6,6-tetramethyl-1-oxopiperidine (31.2 mg, 0.0005 mol) in 20 ml of ethanol, and add dihydrate to it A solution of copper acetate (108.5 mg, 0.0005 mol) in water (50 mL). The resulting light brown solution was stirred in air at room temperature for 30 minutes, then the gray-green precipitate was filtered off, the filtrate was extracted with ethyl acetate, the organic phase was dried and filtered, and the solvent was distilled off under reduced pressure to obtain a brown crude product. The crude product was dissolved in ethanol and slowly volatilized in the air for 2 days to obtain yellow-brown blocky crystals. Yield: 147 mg (85%). UV-Vis spectrum UV-Vis: λ max / nm (1.0 × 10 -5 mol dm -3 , CH 2 Cl 2 ) 270 (ε / 10 3 dm 3 mol −1 cm −1 53.6), 372 (8.5), 430 (6.70), 610 (0.33). Infrared spectrum FT-IR (solid, cm -1 ): 1655s,160s, 1536s, 1442m, 1370s, 1309w, 1...
Embodiment 2
[0023] Embodiment 2: the synthesis of benzaldehyde
[0024] Add the above copper catalyst (13.8 mg, 0.02 mmol, 2%), 2,2,6,6-tetramethyl-1-oxopiperidine (TEMPO) (0.050 mmol, 5%) into a 25 mL round bottom flask , phenylethyl alcohol (108 mg, 1 mole) and 5 mL of deionized water. The reaction was exposed to air at room temperature and stirred for 2 hours. After the resulting solution was extracted with ethyl acetate, the organic phase was washed with water and washed with anhydrous sulfuric acid. Magnesium is dried and easily removed under reduced pressure to obtain benzaldehyde (purity greater than 98%). Yield: 101 mg (95%). H NMR spectrum 1 H NMR (400 MHz, CDCl 3 ) δ 9.92 (s, 1H), 7.83-7.78 (m, 2H), 7.55-7.38 (m, 3H). Mass Spectrum GC-MS (m / z): 106 (calc. 106).
Embodiment 3
[0025] Embodiment 3: the synthesis of 4-methoxybenzaldehyde
[0026] Add the above copper catalyst (13.8 mg, 0.02 mmol, 2%), 2,2,6,6-tetramethyl-1-oxopiperidine (TEMPO) (0.050 mmol, 5%) into a 25 mL round bottom flask , 4-methoxyphenethyl alcohol (138 mg, 1 mmol) and 5 mL of deionized water, the reaction was exposed to air at room temperature and stirred for 2 hours, the resulting solution was extracted with ethyl acetate, and the organic phase was washed with water , dried with anhydrous magnesium sulfate, and easily removed under reduced pressure to obtain 4-methoxybenzaldehyde (purity greater than 98%). Yield: 126 mg (93%). H NMR spectrum 1 H NMR (400 MHz, CDCl 3 ) δ 9.97 (s, 1H), 7.84 (d, J =8.7 Hz, 2H), 7.01 (d, J = 8.8 Hz, 2H), 3.90 (s, 3H, H OCH3 ). Mass Spectrum GC-MS (m / z): 136 (calc. 136).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com