Trichinella spiralis paramyosin h-2d restricted TH epitope p2, composition and application thereof
A composition and technology of Trichinella, applied in the field of immunobiology, can solve problems such as difficulty in correct diagnosis, difficulty in drug treatment, and inability to solve repeated infection of the worm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: Preparation of rTs-Pmy protein
[0054] The Ts-Pmy plasmid bacterial liquid is transformed into Escherichia coli with a full-length plasmid containing the paramyosin gene (Ts-Pmy GenBank accession No.: EF429310), which is equivalent to the patent application with publication number CN100999737A (application number 200710000018.1) The Escherichia coli BL21 transformed with the paramyosin gene (Ts86cDNA) prepared in Example 2 can also refer to this patent application for its preparation method. Refer to this patent application to prepare recombinant Ts-Pmy protein (rTs-Pmy protein).
Embodiment 2
[0055] Embodiment 2: the synthesis of polypeptide sequence
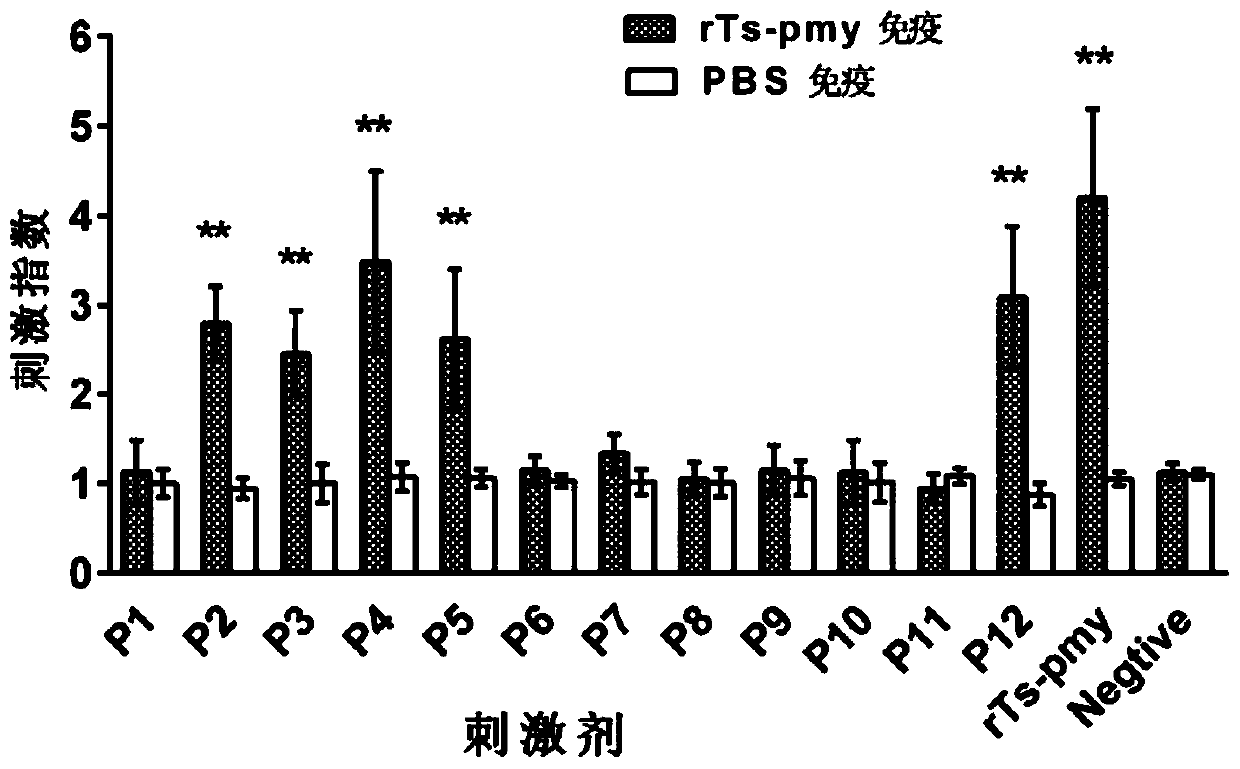
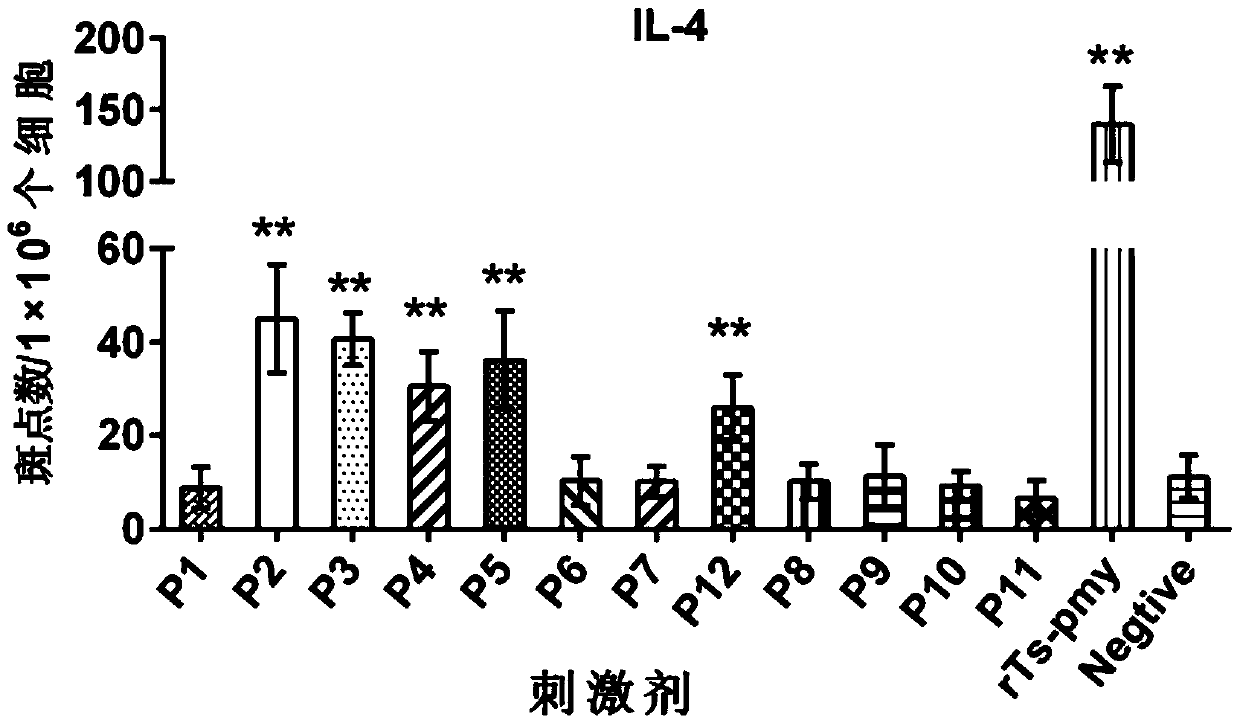
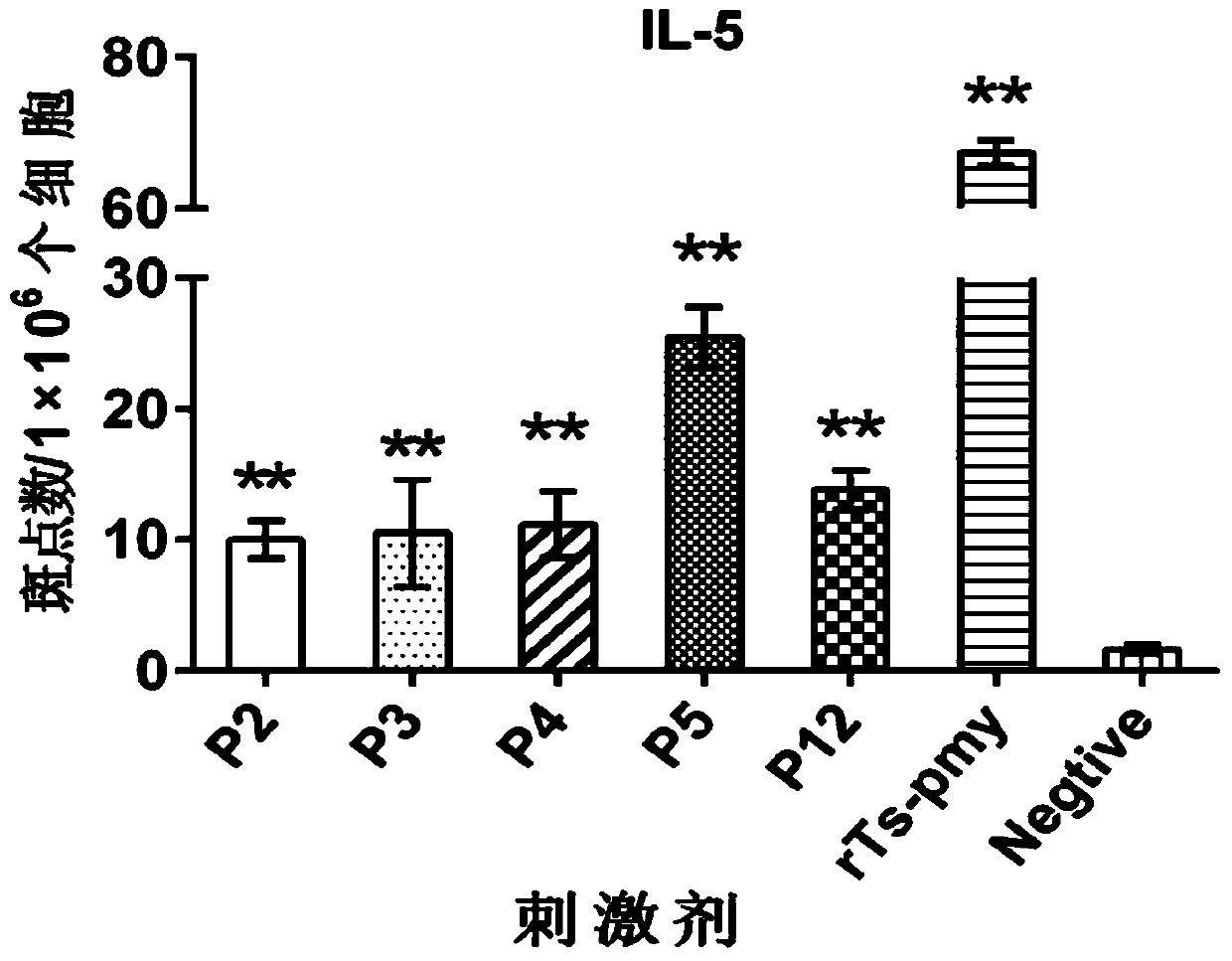
[0056] 12 polypeptide sequences were synthesized according to the amino acid sequence of the recombinant Ts-Pmy protein (rTs-Pmy protein), as shown in Table 1 below. Wherein the position refers to the amino acid position on the rTs-Pmy protein.
[0057] Entrust Beijing Ovia Biotechnology Co., Ltd. to synthesize.
[0058] Table 1: Peptide sequences
[0059]
[0060]
Embodiment 3
[0061] Embodiment 3: In vitro test identification of polypeptide
[0062] 1. Experimental samples:
[0063] The recombinant Ts-Pmy protein (rTs-Pmy) prepared in Example 1.
[0064] Polypeptides P1-P12 synthesized in Example 2.
[0065] 2. Experimental animals and groups:
[0066] BALB / c female mice aged 6-8 weeks were randomly divided into two groups, the recombinant protein group and the PBS control group, with 6 mice in each group.
[0067] 3. Experimental method
[0068] (1) Immunization of animals: 50 μg of rTs-Pmy (dissolved in 50 μl PBS) or 50 μl of PBS plus an equal volume of adjuvant MONTANIDE per mouse TM ISA 50 V2 (Seppic Company), subcutaneously multi-point immunized mice, and boosted immunization with the same dose twice after 2 weeks and 4 weeks.
[0069] (2) Proliferation and isolation of spleen lymphocytes:
[0070] One week after the last immunization, the mice were killed and splenic lymphocytes were isolated (operated according to the instructions of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com