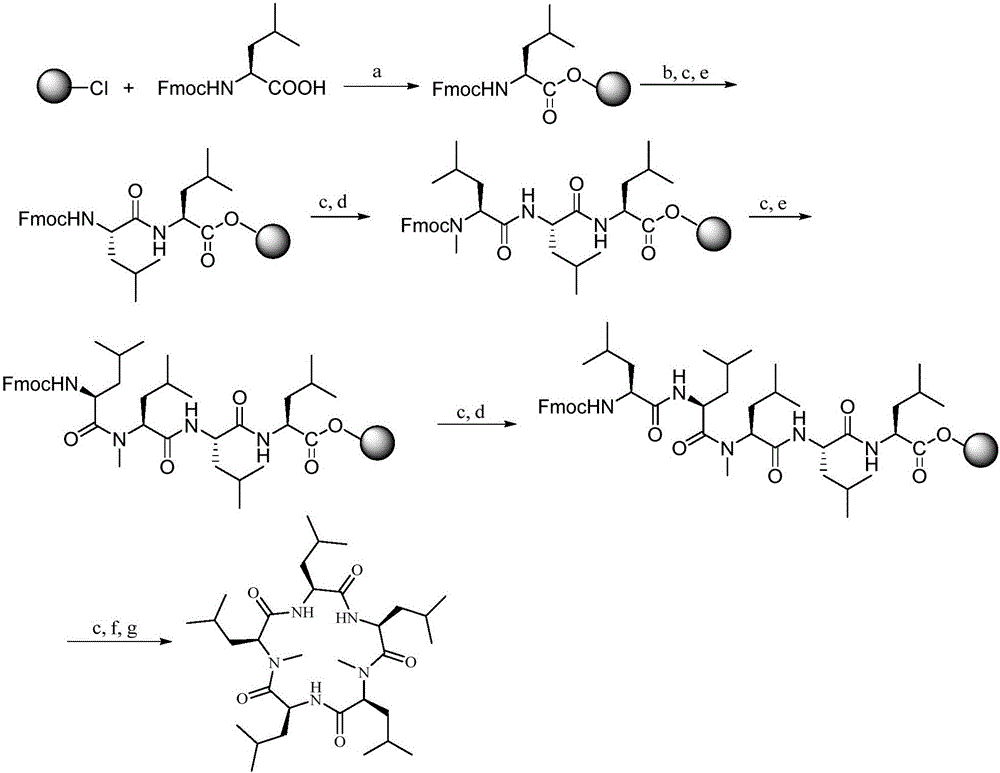
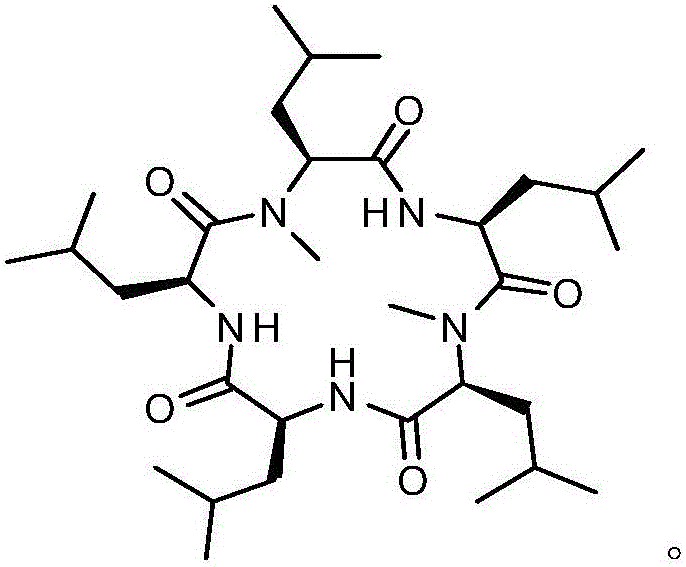
Synthesis method of cyclic pentapeptide
A synthetic method, the technology of cyclic pentapeptide, applied in the field of organic chemical synthesis, can solve the problems of large solvent and time consumption, low yield of cyclization, etc., and achieve the effects of improved purity, low raw material cost, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Synthesis of Fmocyl N-methyl-leucine (Fmoc-N-Me-Leu-OH)
[0037] Fmoc-Leu-OH (7.07g, 2mmol) was suspended in 100mL of toluene, paraformaldehyde (9.00g, 3mmol) and p-toluenesulfonic acid (0.200g, catalytic amount) were added, and the mixture was azeotropically refluxed for 30 minutes to remove water. After the solution was cooled, the solution was washed with saturated NaHCO 3 Wash with aqueous solution (25mL×2) and wash with anhydrous Na 2 SO 4 dry. Filter, carry out silica gel column chromatography (eluent V 石油醚 :V 乙酸乙酯 =8:1), to obtain white compound (1), in a 250mL flask, add compound (1) (7.00g, 2mmol), dichloromethane (150mL), anhydrous aluminum trichloride (4.00g, 3mmol), three Ethylsilane (4.8mL, 3mmol) was reacted for several hours, and when the raw material point basically disappeared as monitored by TLC, the reaction was stopped, and the reaction solution was washed twice with 1mol / L hydrochloric acid. Anhydrous Na for washing solution 2 SO ...
Embodiment 2
[0038] Embodiment 2 fluorenyl methaneoxycarbonyl leucine (Fmoc-Leu-OH) is connected to 2-chlorotrityl chloride resin
[0039] Activation of the resin: Add 1.00 g of 2-chlorotrityl chloride resin (loading capacity: 0.985 mmol / g) to the peptide synthesis tube, soak in 15 mL of dichloromethane for 30 min to fully swell and activate the resin, and then drain the liquid.
[0040] Fmoc-Leu-OH is connected to the resin: add Fmoc-Leu-OH (1.06g, 3mmol) in a 50mL Erlenmeyer flask, dissolve it with 20mL DCM (dichloromethane), then add DIEA to it to adjust the pH to about 8, After stirring for 1 minute, the mixed solution was added into the reactor containing the activated resin, nitrogen gas was introduced, and then the reaction was performed on a shaking table for 4 hours. After the reaction was completed, the reaction liquid was drained, and the resin was washed three times with 10 mL of DCM and DMF (dimethylformamide) respectively, and the washing liquid was drained.
Embodiment 3
[0041] The sealing of the residual active site of embodiment 3 resin
[0042] Take 18mL of methanol, dissolve 2mL of DIEA in methanol to make a solution of DIEA / MeOH (1:9), add the resin obtained in Example 2 to which the first amino acid has been connected, and react for 30min. After the blocking reaction, the resin was washed three times with 10 mL DCM and DMF respectively, and the washing solution was drained. The complex Fmoc-Leu-Resin (R-1A) of protected amino acid and resin was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com