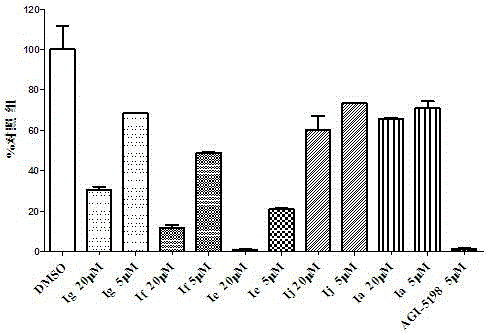
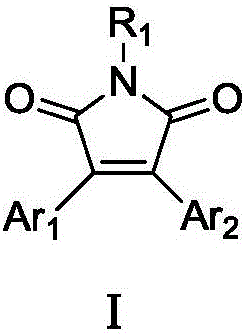
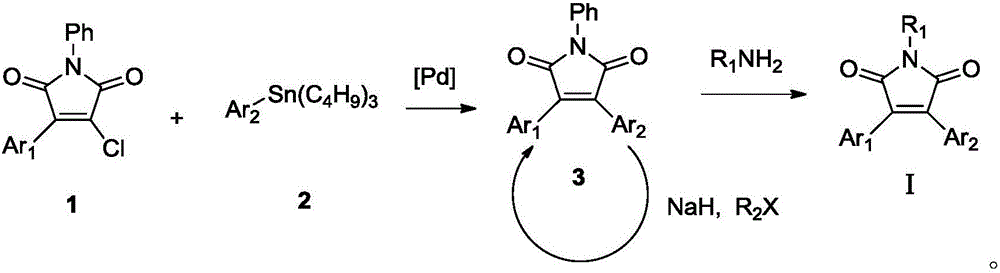
Double-aryl maleimide compound, pharmaceutically acceptable salt thereof, method for preparing double-aryl maleimide compound and pharmaceutically acceptable salt and application of double-aryl maleimide compound and pharmaceutically acceptable salt
A maleimide and compound technology, applied in the field of bisarylmaleimide compounds, can solve problems such as tumors and activity decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: 1-methyl-3-(tributyltinyl)-1H-pyrazol[3,4-b]pyridine (2a)
[0029] Add 2.0g (4.9mmol) 3-(tributyltinyl)-1H-pyrazol[3,4-b]pyridine and 30mL anhydrous DMF into the three-necked flask, control the temperature at 0-5°C, and add 0.22g in batches (5.5mmol) 60% NaH, after adding, keep stirring for 30min, then dropwise add 0.84g (5.9mmol) methyl iodide, react at 0~5°C for 1h after dropping, then rise to room temperature for 0.5h, after the reaction, put The reaction solution was poured into 600mL water, extracted with dichloromethane (50mL×3), the organic layers were combined, washed with saturated brine (150mL×3), dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column Purification by analysis (petroleum ether: ethyl acetate = 6:1, v / v) gave 1.56 g of oil 2a with a yield of 75.5%. 1 HNMR (CDCl 3 )δ:0.89(9H,t,J=7.5Hz),1.21-1.26(6H,m),1.33-1.38(6H,m),1.58-1.63(6H,m),4.19(3H,...
Embodiment 2
[0030] Example 2: 1-Ethyl-3-(tributyltinyl)-1H-pyrazol[3,4-b]pyridine (2b)
[0031] The synthesis method is the same as that in Implementation 1, except that ethyl bromide is used instead of methyl iodide to obtain oil 2b, yield: 62.3%, 1 HNMR (CDCl 3 )δ:0.89(9H,t,J=7.5Hz),1.22-1.27(6H,m),1.34-1.40(6H,m),1.54(3H,t,J=7.5Hz),1.57-1.64(6H ,m),4.63(2H,q,J=7.5Hz),7.04-7.07(1H,m),8.00(1H,dd,J=8.0,1.5Hz),8.50(1H,dd,J=4.5,1.5 Hz).
Embodiment 3
[0032] Example 3: 1-Butyl-3-(tributyltinyl)-1H-pyrazol[3,4-b]pyridine (2c)
[0033] The synthetic method is the same as that in Implementation 1, except that methyl bromide is used instead of methyl iodide to obtain oil 2c with a yield of 58.3%. 1 HNMR (CDCl 3 )δ:0.89(9H,t,J=7.5Hz),0.95(3H,t,J=7.5Hz),1.20-1.29(8H,m),1.32-1.39(8H,m),1.56-1.63(6H ,m),4.57(2H,t,J=7.5Hz),7.03-7.08(1H,m),8.01(1H,dd,J=8.0,1.5Hz),8.50(1H,dd,J=4.5,1.5 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com