Method for preparing hydrogen-rich gas through gas-solid synchronous gasification of pyrolysis gas and biomass charcoal of biomass
A technology of biomass charcoal and pyrolysis gas, which is applied in the direction of manufacturing combustible gas, treating combustible gas with catalysis, and gasification technology to achieve the effects of high carbon conversion rate, high utilization rate and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
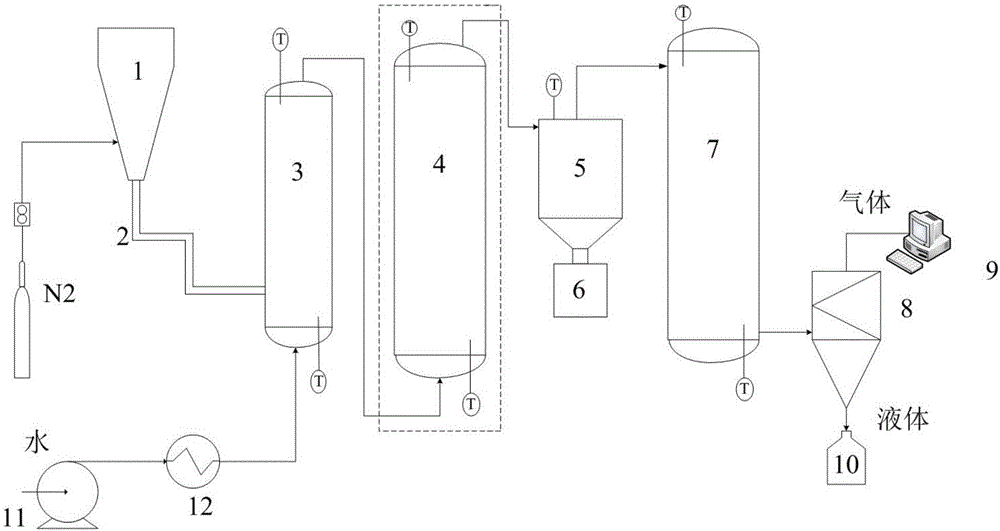
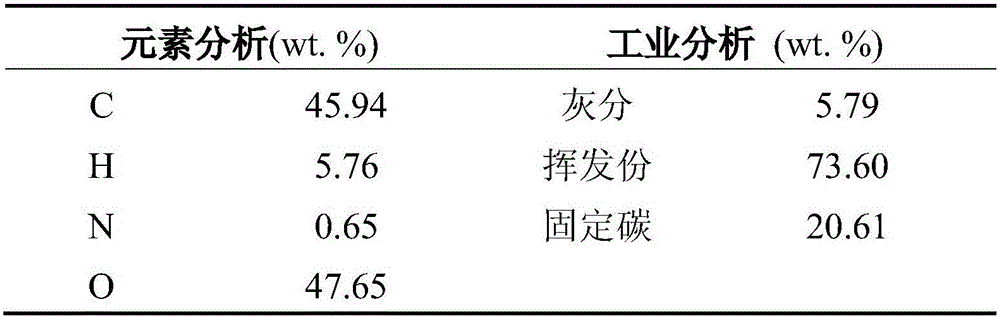
[0034] The raw biomass is put into the hopper 1, and sent into the fluidized bed reactor 3 through the screw feeder 2 (the mass flow rate is 200g / h) for rapid pyrolysis. In the bed reactor 3, S / B=3. The pyrolysis gas and biochar generated by the rapid pyrolysis of biomass enter the entrained bed reactor 4 with the air flow for synchronous gasification reaction of the cracked gas and biochar. The temperature of the entrained bed reactor 4 is 700-850°C (ΔT = 50°C, divided into four separate tests). The product from entrained bed reactor 4 enters cyclone separator 5 to remove ash and then enters fixed bed reactor 7 for catalytic reforming reaction, WHSV is 1, and catalytic reforming reaction temperature is 850°C.
[0035] The reaction results are shown in Table 2 below:
[0036] Table 2
[0037]
Embodiment 5-8
[0039] The raw biomass is put into the hopper 1, and sent into the fluidized bed reactor 3 through the screw feeder 2 (the mass flow rate is 200g / h) for rapid pyrolysis. In the bed reactor 3, S / B=1-4, four experiments were carried out separately. The pyrolysis gas and biochar generated by the rapid pyrolysis of biomass enter the entrained bed reactor 4 with the airflow for synchronous gasification reaction of the pyrolysis gas and biochar. The temperature of the entrained bed reactor 4 is 800°C. The product from the reactor 4 enters the cyclone separator 5 to obtain residual solids, and the condensable gases condense to obtain tar.
[0040] The reaction results are shown in Table 3 below:
[0041] table 3
[0042]
[0043]
Embodiment 9-12
[0045] The raw biomass is put into the hopper 1, and sent into the fluidized bed reactor 3 through the screw feeder 2 (the mass flow rate is 200g / h) for rapid pyrolysis. In the bed reactor 3, S / B=3. The pyrolysis gas and biochar generated by the rapid pyrolysis of biomass enter the entrained bed reactor 4 with the air flow for synchronous gasification of the pyrolysis gas and biochar. The temperature of the entrained bed reactor 4 is 850°C. The product from entrained bed reactor 4 enters cyclone separator 5 to remove ash and then enters fixed bed reactor 7 for catalytic reforming reaction, WHSV is 1, catalytic reforming reaction temperature is 700-850°C, and is carried out separately Four experiments were performed, each with a temperature difference of 50 degrees.
[0046] The reaction results are shown in Table 4 below:
[0047] Table 4
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com