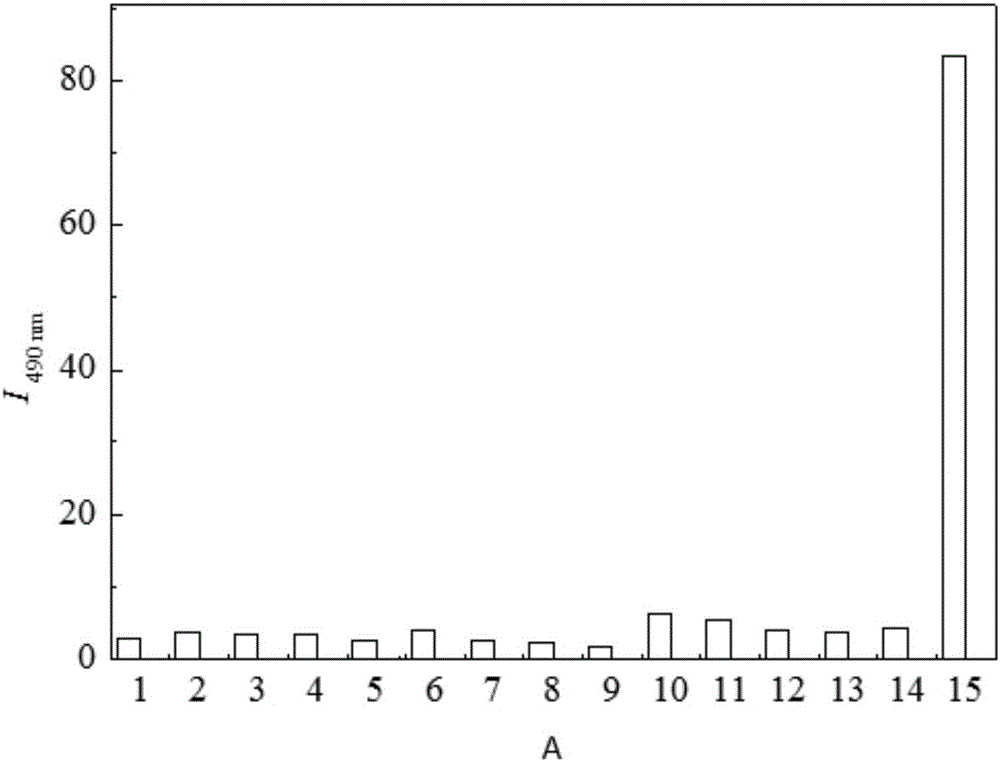
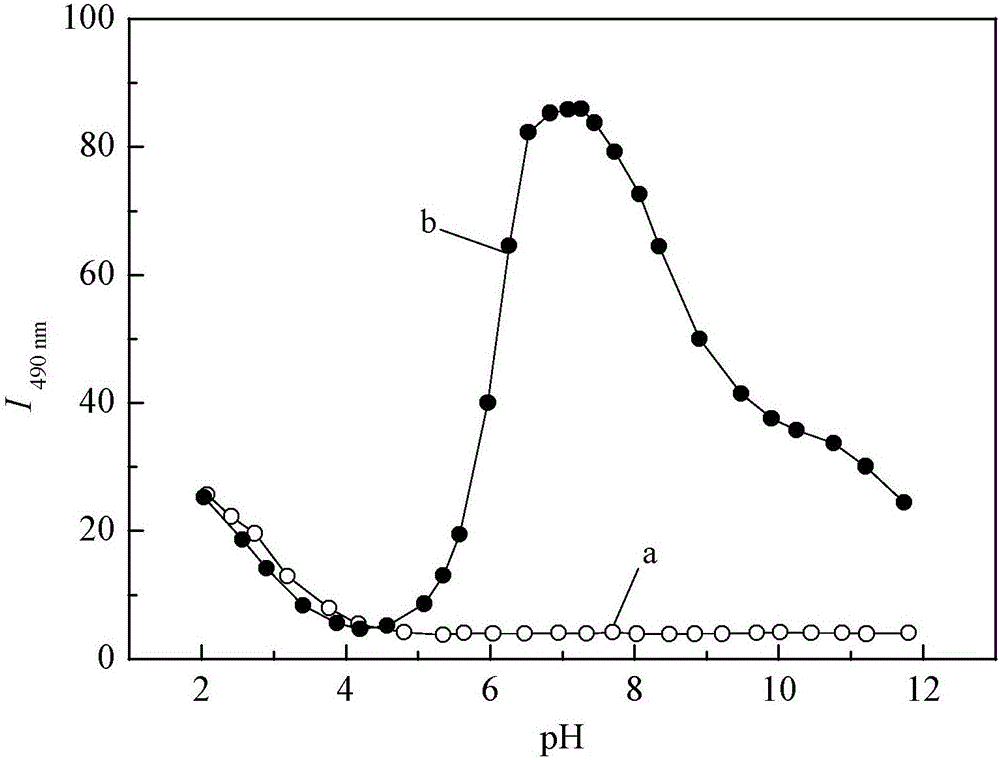
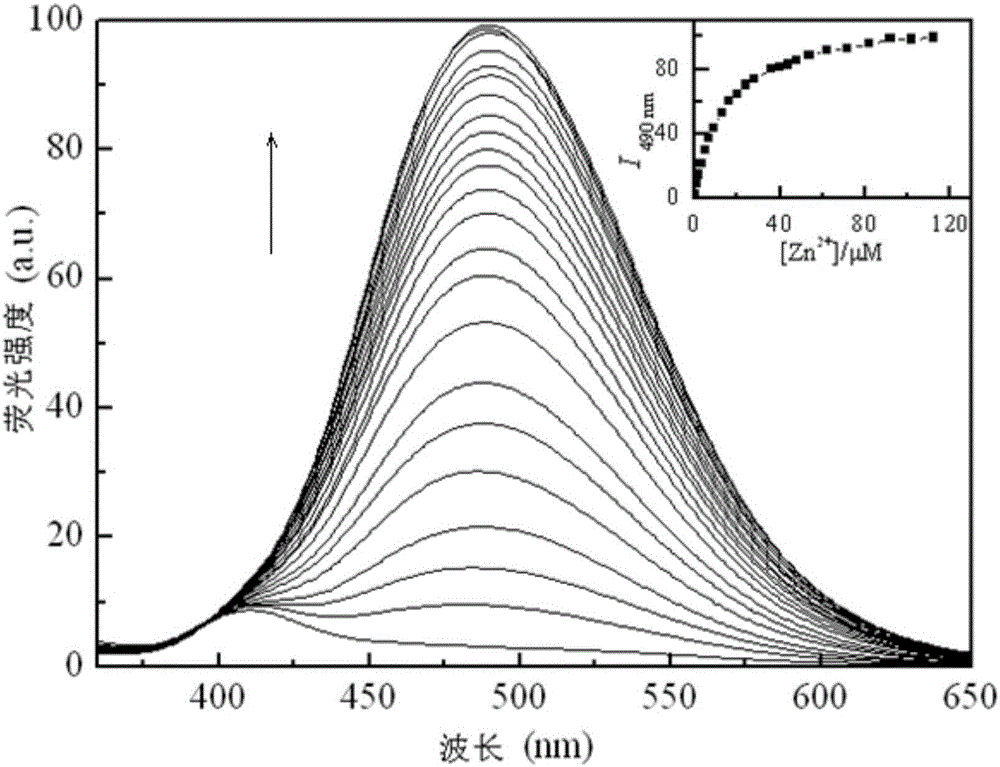
Oxalyl aminoquinoline derivative fluorescent molecular probe and preparation method and application thereof
A fluorescent molecular probe, oxamidoquinoline technology, which is applied in the field of biological fluorescent molecular probes, can solve the problems of many kinds of synthetic raw materials, harsh synthetic conditions, etc., and achieves the advantages of not harsh conditions, simple preparation method and easy-to-obtain raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0026] Specific embodiment one: The general structural formula of the oxalamidoquinoline derivative fluorescent molecular probe of the present embodiment is:
[0027] wherein R is alkoxy, amino, alkylamino, arylamino or hydroxyalkyl.
specific Embodiment approach 2
[0028] Specific embodiment two: the preparation method of the fluorescent molecular probe of oxamidoquinoline derivative described in specific embodiment one is as follows:
[0029] 1. Mix 8-aminoquinoline and diethyl oxalate according to the molar ratio of 1: (5-10), heat up to 150-220°C, and react for 3-12 hours to obtain a reaction mixture. After the reaction mixture is purified, Obtain the intermediate, represented by EOQ;
[0030] 2. Mix EOQ and amine according to the molar ratio of 1: (2~10), heat up to 80~150°C, react for 2~12 hours to obtain crude product I; after purifying the obtained crude product I, obtain monosubstituted oxalyl Aminoquinoline derivative fluorescent molecular probe; the amine is ammonia, organic primary or secondary amine.
specific Embodiment approach 3
[0031] Embodiment 3: The difference between this embodiment and Embodiment 2 is that the molar ratio of 8-aminoquinoline to diethyl oxalate is 1:8. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com