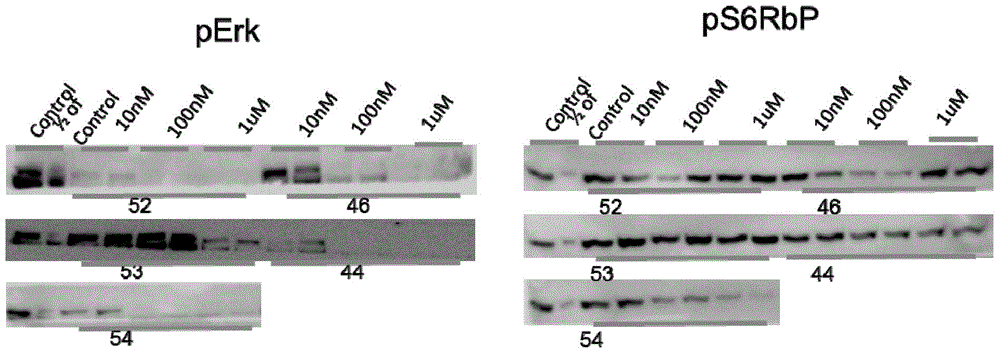
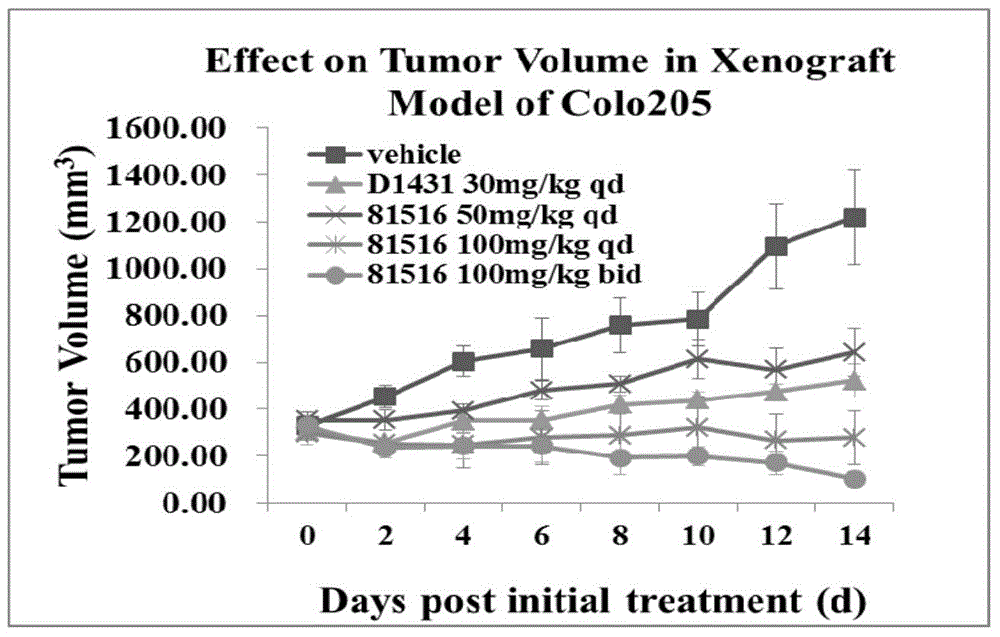
Sulfonamide aryl acetylene compound and use thereof
A technology of aryl alkyne and sulfonamide, applied in the field of sulfonamido aryl alkyne compounds, can solve the problems of anemia, damage, leukopenia and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] N-(2,4-difluoro-3-((3-methoxy-1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)phenyl)pyridine-3-sulfonamide
[0166] N-(2,4-difluoro-3-((3-methoxy-1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)phenyl)pyridine-3-sulfonamide .
[0167]
[0168] Synthesize according to the route of scheme A below:
[0169]
[0170] Step 1: Synthesis of 5-bromo-3H-pyrazolo[3,4-b]pyridin-3-one
[0171] In a 1000ml flask, add 50g (200mmol) of 5-bromo-2-chloronicotinic acid methyl ester, 500ml ethanol, 800mmol of 80% hydrazine hydrate, and reflux for 12h under stirring. During the reaction, solids are precipitated. After the reaction, cool to room temperature , add 1000ml of ice water, wait for the solid to fully separate out and then suction filter, the filter cake is fully washed with water and then vacuum-dried to obtain 36g of light yellow solid with a yield of 85%. Product MS (ESI), m / z: 212, 214 (M + +H + ).
[0172] Step 2: Synthesis of 5-bromo-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridin-...
Embodiment 2
[0189] N-(2,4-difluoro-3-((3-methoxy-1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)phenyl)propane-1-sulfonamide
[0190] N-(2,4-difluoro-3-((3-methoxy-1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)phenyl)propane-1-sulfonamide
[0191]
[0192] Synthesize according to the route of scheme B below:
[0193]
[0194] Step 10: Synthesis of N-(2,4-difluorophenyl)propane-1-sulfonamide
[0195] In a 250ml round-bottomed flask, dissolve 3g (19.6mmol) 3-ethynyl-2,4-difluoroaniline (prepared according to the above method) in 150ml dichloromethane, drop 3g (21.5mmol, 1.1eq) propanesulfonyl chloride, 2.3ml (29.4mmol, 1.5eq) pyridine, catalytic amount of 4-N,N dimethylaminopyridine, reflux overnight. After the reaction was detected by thin-layer chromatography, add 5ml of 6N hydrochloric acid and appropriate amount of water, extract three times with dichloromethane, wash the organic phase with dilute hydrochloric acid and saturated saline once, dry with anhydrous sodium sulfate, evaporate the sol...
Embodiment 3
[0200] N-(3-((1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)phenyl)propane-1-sulfonamide
[0201] N-(3-((1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)phenyl)propane-1-sulfonamide
[0202]
[0203] The synthesis method is as in Example 2.
[0204] Product characterization: 1 HNMR (400MHz, DMSO-d 6 ):δ(ppm)13.91(s,1H),9.98(s,1H),8.69(d,J=2Hz,1H),8.50(d,J=1.8Hz,1H),8.20(s,1H), 7.38-7.42(m,2H),7.32(d,J=7.8Hz,1H),7.27(dd,J=1.9,7.1Hz,1H),3.12(t,J=7.6Hz,2H),1.67-1.74 (m,2H),0.95(t,J=7.4Hz,3H).MS(ESI),m / z:341(M + +H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com