Ferulic acid derivatives, preparation and application of ferulic acid derivatives
A technology of ferulic acid and derivatives is applied in the application field of ferulic acid derivatives in the prevention and/or treatment of stroke-related diseases, and can solve the problems of limited treatment means and great harm of stroke.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
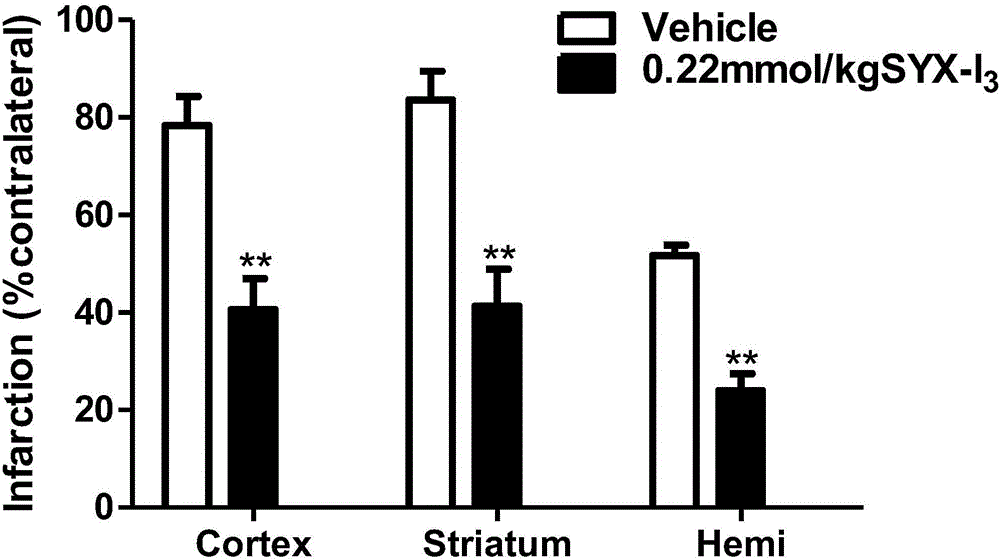
Image
Examples
preparation example Construction
[0038] The present invention has no special limitation on the preparation method of the ferulic acid derivatives, the preparation method of this type of compound known to those skilled in the art can be used, and those skilled in the art can select and adjust according to the actual situation and product requirements. , the ferulic acid derivatives described in the present invention are preferably ester compounds of ferulic acid and ADTO (CH 2 ) z X (z = 3 ~ 10, X is a halogen) obtained after the reaction, specifically ethyl ferulate and ADTO (CH 2 ) z Obtained after Br reaction.
[0039] The present invention provides an application of the ferulic acid derivative described in any one of the above technical solutions in the preparation of drugs for the prevention and / or treatment of cerebral apoplexy.
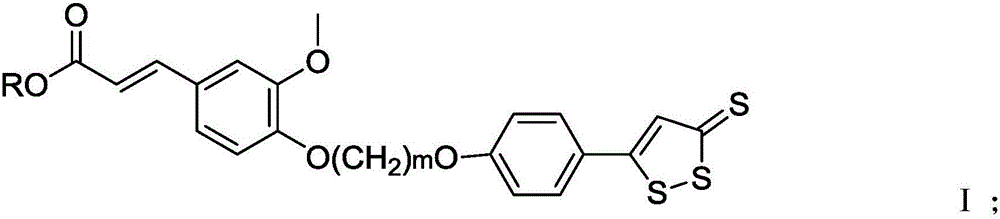
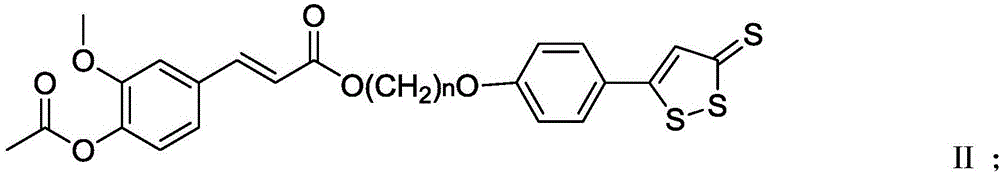
[0040] In the present invention, the ferulic acid derivative is preferably the above-mentioned compound having the structure of formula I or formula II, more specifically 3-...
Embodiment 1
[0054] 3-Methoxy-4-{3-[4-(3H-1,2-dithiol-3-thiol-5-yl)phenoxy]propoxy}ethyl phenylacrylate ( SYX-I 1 )Synthesis
[0055] (E)-Ethyl 4-hydroxy-3-methoxyphenylacrylate (222 mg, 1 mmol), 5-[4-(3-bromopropoxy)-phenyl]-[1,2]-di Thiolene-3-thione (347mg, 1mmol), KI (17-34mg, 0.1-0.2mmol), and potassium carbonate (139mg, 1mmol) were added to acetone, and refluxed at 100°C for 8h. After the reaction, filter with suction, mix the sample to pass through the column, perform column chromatography, and eluate (petroleum ether: ethyl acetate = 8:1). A yellow powdery solid was obtained. Yield 68%.
[0056] 1 HNMR (400MHz, CDCl 3 ), 7.06(s,1H,ArH),6.99(d,J=8.8Hz,2H,ArH),6.90(d,J=8.2Hz,1H,ArH),6.31(d,J=15.9Hz,1H,C= CH),4.29–4.21(m,6H,OCH 2 ),3.88(s,3H,OCH 3 ),2.36(p,J=6.0Hz,2H,CH 2 ), 1.34(t, J=7.1Hz, 3H, CH 3 ).
[0057] 13 CNMR (400MHz, CDCl 3 )δ215.10,172.96,167.17,162.17,150.22,149.53,144.35,134.62,128.56,127.79,124.21,122.39,116.15,115.46,112.69,110.09,65.23,64.78,60.38,55.8...
Embodiment 2
[0060] Ethyl 3-methoxy-4-{4-[4-(3H-1,2-dithiol-3-thiol-5-yl)phenoxy]butoxy}phenylacrylate ( SYX-I 2 )Synthesis
[0061] Prepared with reference to the method of Example 1 from ethyl ferulate and 5-(4-(4-bromobutoxy)phenyl)-1,2-dithiole-3-thione to obtain a yellow powder shaped solid. Yield 65%.
[0062] 1 HNMR (400MHz, CDCl 3 ), 7.05(d, J=1.8Hz, 1H, ArH), 6.96(d, J=8.8Hz, 2H, ArH), 6.87(d, J=8.3Hz, 1H, ArH), 6.31(d, J=15.9Hz ,1H,C=CH),4.26(q,J=7.1Hz,2H,OCH 2 ), 4.13 (q, J=5.6Hz, 4H, OCH 2 ),3.88(s,3HOCH 3 ),2.12–1.99(m,4H,CH 2 ), 1.34(t, J=7.1Hz, 3H, CH 3 ).
[0063] 13 CNMR (400MHz, CDCl 3 )δ215.08,173.04,167.20,162.34,150.37,149.47,144.42,134.56,128.54,127.54,124.04,122.45,116.00,115.40,112.38,110.00,68.41,67.91,60.37,55.88,25.91,25.67,14.34.
[0064] LC-MS: Calcd.ForC 25 h 26 o 5 S 3 [M+H] + 503.1021, Found: 503.1037.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com