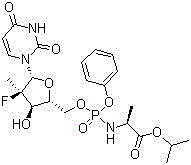
Preparation method of sofosbuvir
A technology of sofosbuvir and pentafluorophenol, applied in the field of preparation of anti-HCV drugs, can solve the problems of environmental impact, no mention of pentafluorophenol recovery and utilization, etc., achieves easy operation, easy control of impurities, and reduced impact Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation method of Sofosbuvir of the present invention comprises the following steps:
[0025] Preparation of Sofosbuvir:
[0026]
[0027] In the reaction flask, under anhydrous and oxygen-free conditions, add 120g (2'R)-2'-deoxy-2'-fluoro-2'-methyluridine, 600mL tetrahydrofuran, stir, cool down to -30°C, Add 268g of 1.7M tert-butylmagnesium chloride dropwise; after dropping, stir the reaction for 1 hour, then raise the temperature to -10°C, add dropwise 230g of N-[(S)-(2,3,4,5,6-pentafluorophenoxy ) 500 mL tetrahydrofuran solution of phenoxyphosphoryl]-L-alanine isopropyl ester; after dropping, react at -10°C for 1 hour, then warm up to room temperature for reaction, and monitor the reaction by TLC. After the reaction is complete, cool down to 0°C, and start to drop concentrated hydrochloric acid / water (100mL / 600mL) to quench the reaction; distill off THF under reduced pressure, then add 800mL of diethyl ether, stir and separate the layers, and extract the ...
Embodiment 2
[0034] The preparation method of Sofosbuvir of the present invention comprises the following steps:
[0035] Preparation of Sofosbuvir:
[0036]
[0037] In the reaction flask, under anhydrous and oxygen-free conditions, add 95g (2'R)-2'-deoxy-2'-fluoro-2'-methyluridine, 500mL tetrahydrofuran, stir, cool down to -30°C, Add 213g of 2.0M isopropylmagnesium chloride dropwise; after dropping, stir the reaction for 1 hour, then raise the temperature to -10°C, add dropwise 198g of N-[(S)-(2,3,4,5,6-pentafluorophenoxy ) 400 mL tetrahydrofuran solution of phenoxyphosphoryl]-L-alanine isopropyl ester; after dropping, react at -10°C for 1 hour, then warm up to room temperature for reaction, and monitor the reaction by TLC. After the reaction is complete, cool down to 0°C, and start to drop concentrated hydrochloric acid / water (200mL / 500mL) to quench the reaction; distill off tetrahydrofuran under reduced pressure, then add 600mL of diethyl ether, stir and separate the layers, and ex...
Embodiment 3
[0044] The preparation method of Sofosbuvir of the present invention comprises the following steps:
[0045] Preparation of Sofosbuvir:
[0046]
[0047] In the reaction flask, under anhydrous and oxygen-free conditions, add 140g (2'R)-2'-deoxy-2'-fluoro-2'-methyluridine, 600mL dimethyl sulfoxide, stir, and cool down to -30°C, add 339g of 2.0M isopropylmagnesium chloride dropwise; after dropping, stir the reaction for 1 hour, then raise the temperature to -10°C, add dropwise 50g of N-[(S)-(2,3,4,5,6-penta Fluorophenoxy)phenoxyphosphoryl]-L-alanine isopropyl ester in 500mL dimethyl sulfoxide solution; dropwise, react at -10°C for 1 hour, then warm to room temperature for reaction, TLC monitors the reaction . After the reaction is complete, cool down to 0°C and start to drop concentrated hydrochloric acid / water (250mL / 700mL) to quench the reaction; distill off dimethyl sulfoxide under reduced pressure, then add 800mL ether, stir and separate the liquids, and wash the water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com