Antibody phosphorylated at the 74th tyrosine residue of TOPK and its preparation method and application
A tyrosine residue, phosphorylation technology, applied in anti-enzyme immunoglobulins, instruments, measuring devices, etc., can solve the problem of inability to accurately reflect TOPK activity, slow research in the field of inhibitors, and hinder the development of clinical disease diagnosis and treatment prospects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
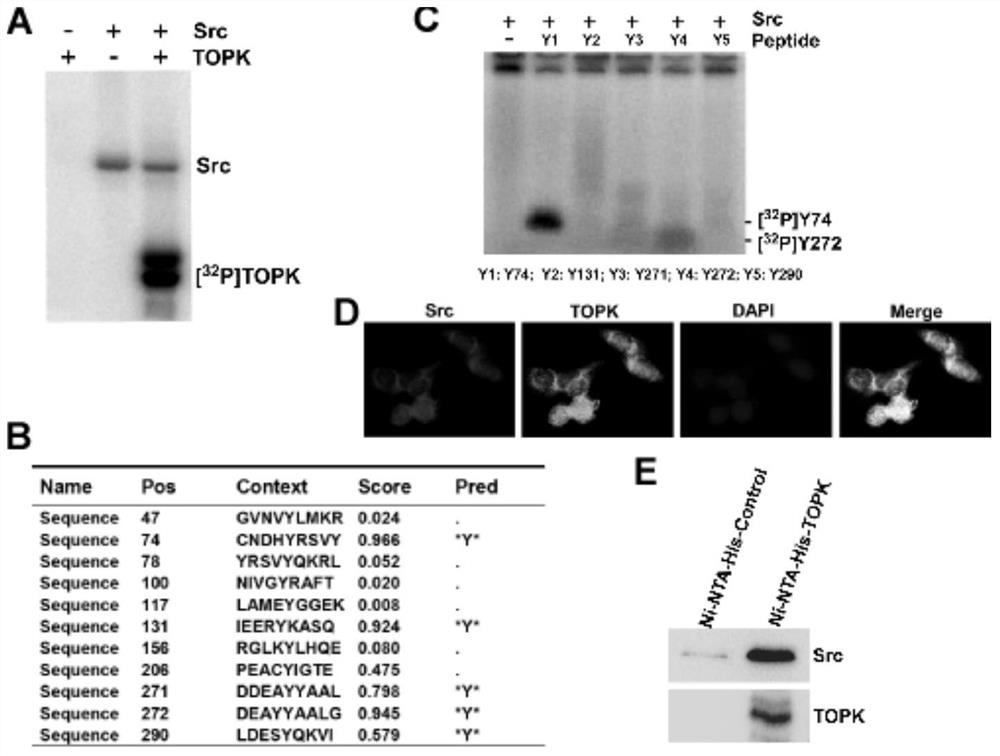
[0091] Example one determines that Src is toPK upstream protein kinase, phosphorylated TOPK at the Y74 and Y272 sites.
[0092] 1. Active Src phosphorylated TOPK was found by in vitro kinase experiments.
[0093] The specific steps of in vitro kinase experiments are as follows:
[0094] 1.1 Expression and purification of TOPK protein in E.coli BL21 bacteria: pET46-His-TOPK-WT plasmid was converted into E.coli BL21 competent cells, and single colonies were cultured overnight at 37 °C. The bacteria were inoculated again overnight, incubated at 37 °C to OD600 to 0.6~0.8, then added 1mM IPTG 30 °C oscillation induction for 4h, followed by repeated freeze-thawing of bacterial bodies, added Ni-NTA-His-binding beads (purchased from QIAGEN), incubated at 4 °C overnight, and then washed with PNI 20 and PNI 40 respectively, and finally PNI 400 elution, quantification, electrophoresis, And Coomassie bright blue staining identification.
[0095] 1.2 We incubate the prepared TOPK protein 2ug, ...
Embodiment 2
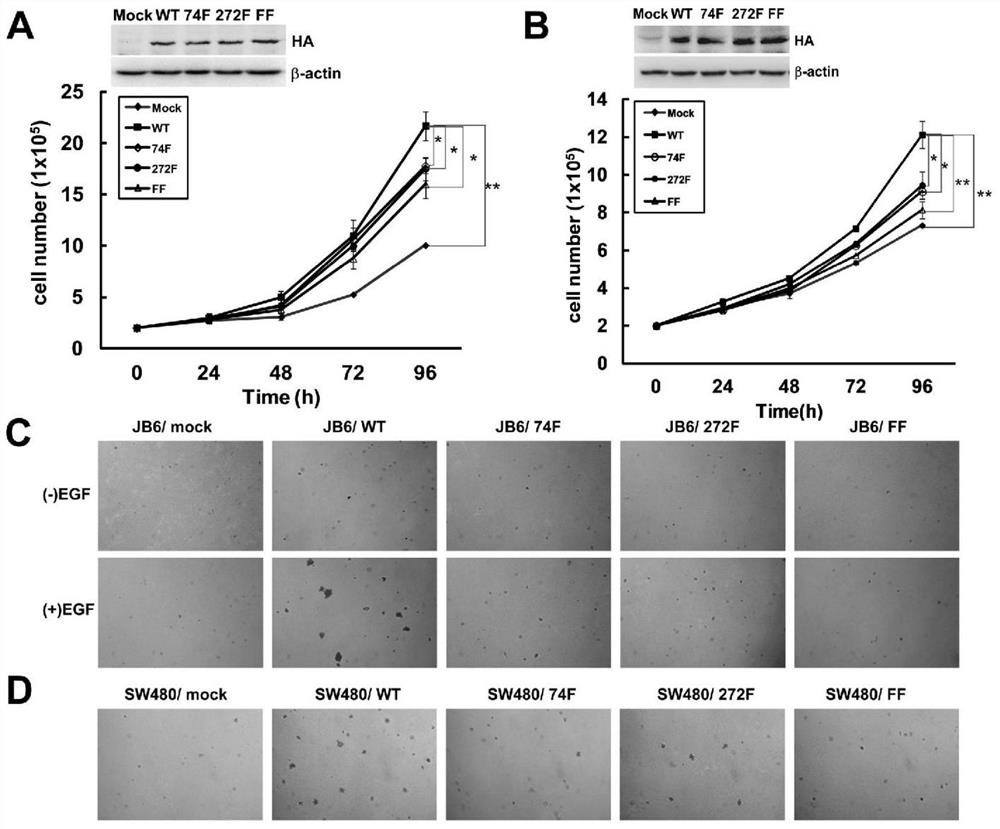
[0122] Example 2 explores the effect of Src phosphorylation on TOPK function.
[0123] 1. Single mutation and double mutation of THE Y74 and Y272 sites of TOPK are carried out by subclonal technology.
[0124] First of all, according to the requirements of the two sites of tyrosine mutation to phenylalanine, the corresponding primers were designed and synthesized (synthesized by Shanghai Yingjun Biotechnology Co., Ltd.), and then PCR was performed with pcDNA3-HA-TOPK as a template, and the mutant products were obtained: single-mutant pcDNA3-HA-TOPK (Y74F), pcDNA3-HA-TOPK (Y272F) and double-mutant pcDNA3-HA-TOPK (FF), and then converted to TOP10, and the plasmid was extracted after amplification.
[0125] 2. Establish overexpression stable cell lines in JB6 and SW480 cell lines, and make growth curves to compare the proliferation changes of each group of cells.
[0126] 2.1 PcDNA3.1, pcDNA3-HA-TOPK (WT), pcDNA3-HA-TOPK (Y74F), pcDNA3-HA-TOPK (Y272F), pcDNA3-HA-TOPK (Y272F) and pcDNA...
Embodiment 3
[0148] Example Preparation of three TOPK phosphorylated antibodies.
[0149] 1. According to the TOPK target sequence and the known phosphorylation sites Y74 and Y272, design the phosphorylated and non-phosphorylated polypeptide sequences that need to be synthesized.
[0150] p-TOPK(Y74):NH 2 -Cys Asn Asp His p[Tyr]Ser Val Tyr Gln Lys Arg Leu MetAsp Glu-CONH 2
[0151] NP-TOPK(Y74):NH 2 -Cys Asn Asp His Tyr Ser Val Tyr Gln Lys Arg Leu MetAsp Glu-CONH 2
[0152] p-TOPK(Y272):NH 2 -Cys Asp Glu Ser Asp Phe Asp Asp Glu Ala Tyr p[Tyr]Ala-CONH 2
[0153] NP-TOPK(Y272):NH 2 -Cys Asp Glu Ser Asp Phe Asp Asp Glu Ala Tyr TyrAla-CONH 2
[0154] 2. Prepare phosphorylated antibodies.
[0155] The specific steps are as follows:
[0156] 2.1 According to the design sequence, the polypeptide is synthesized by solid phase synthesis method, purified with RP-HPLC conditions, and identified by LC / MS, with a purity of >85%.
[0157] 2.2 Use KLH carrier protein coupling to obtain polypeptides for a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com