Dabigatran cyclic derivative and its preparation method and use
A technology for dabigatran and derivatives, which is applied in the field of preparing thrombin inhibitors, can solve the problems of low oral bioavailability and the like, and achieve the effects of good bioavailability, good transmembrane absorption, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
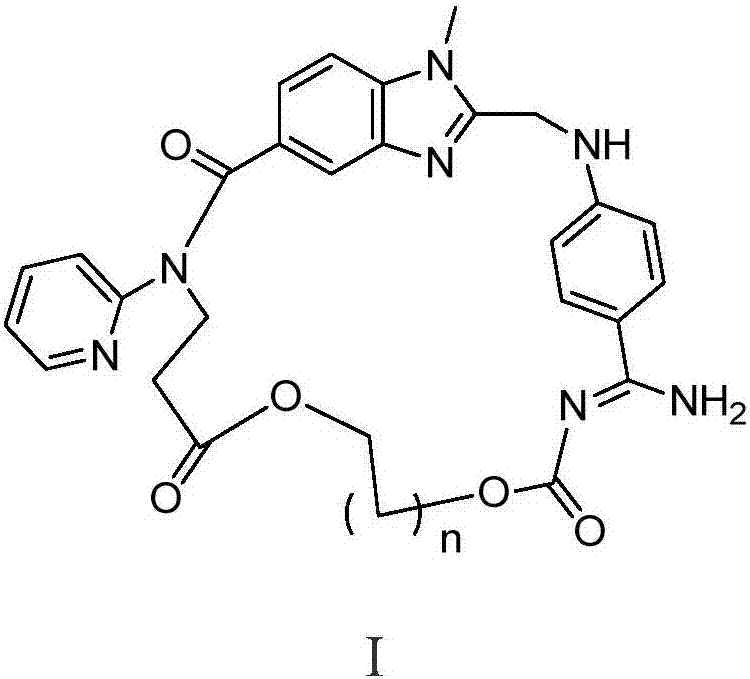
[0053] Dabigatran cyclic derivatives (Ⅰ 1 )preparation
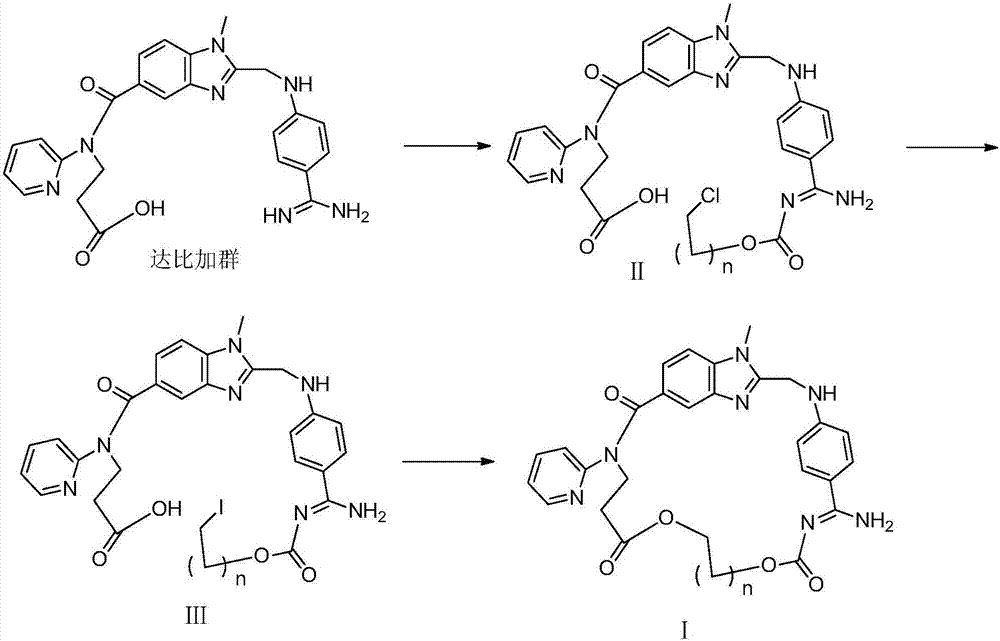
[0054] 1) (Z)-3-(2-(((4-(N'-((2-chloroethoxy)carbonyl)amidino)phenyl)amino)methyl)-1-methyl-N- (Pyridin-2-yl)-1H-benzimidazole-5-carboxamido)propionic acid (Ⅱ 1 )Synthesis.
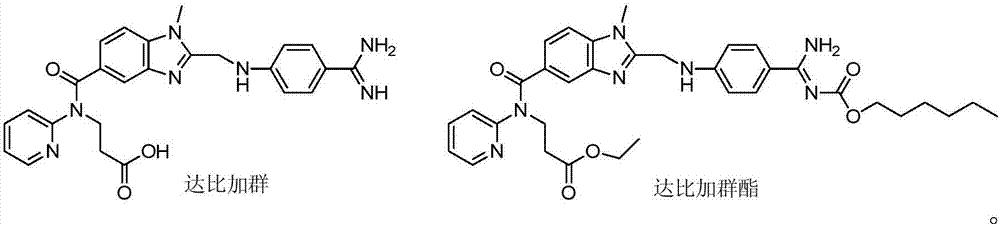
[0055] Chloromethyl chloroformate (0.27g, 2.12mmol) was added dropwise to a solution of dabigatran (1.0g, 2.12mmol) and N-methylmorpholine (0.2ml) in chloroform (10ml), stirred at 0°C for 1 hours, then warmed up to room temperature for 24 hours. Add saturated brine (10mLx3) to wash, dry over anhydrous sodium sulfate and filter, the filtrate is concentrated to dryness, and the residue is purified by column chromatography to obtain light yellow solid II 1 0.86g, yield 71.9%, 1 H NMR (400MHz, DMSO-d6): δ2.66(t, J=6.8Hz, 2H, CH 2 ),3.73(s,3H,NCH 3 ), 4.18(t, J=6.8Hz, 2H, CH 2 ),4.43(s,2H,CH 2 ),6.11(s,2H,CH 2 ),6.79(d,J=8.8Hz,2H,ArH),6.85(d,J=8.8Hz,1H,ArH),7.14(m,2H,ArH),7.43(d,J=8.8Hz,2H, ArH), 7.48(d, J=8.8Hz, 2H, ArH), 7.58(m, 1H, ArH), 8...
Embodiment 2
[0062] Dabigatran cyclic derivatives (Ⅰ 2 ), other conditions are with embodiment 1.
[0063] White solid, yield 45.2%, 1 H NMR (400MHz, DMSO-d6): δ2.60(t, J=6.8Hz, 2H, CH 2 ),3.72(s,3H,NCH 3 ), 4.15(t, J=6.8Hz, 2H, CH 2 ), 4.23(t, J=6.8Hz, 2H, CH 2 ),4.33(s,2H,CH 2 ), 4.47(t, J=6.8Hz, 2H, CH 2 ),6.78(d,J=8.8Hz,2H,ArH),6.83(d,J=8.8Hz,1H,ArH),7.18(m,2H,ArH),7.44(d,J=8.8Hz,2H, ArH), 7.423 (d, J=8.8Hz, 2H, ArH), 7.57 (m, 1H, ArH), 8.33 (m, 1H, ArH), 8.92 (br s, 2H, NH 2 ); ESI-MS: m / z 542[M+H] + .
Embodiment 3
[0065] Dabigatran cyclic derivatives (Ⅰ 3 ), other conditions are with embodiment 1.
[0066] White solid, yield 33.8%, 1 H NMR(400MHz,DMSO-d6):δ1.85(m,2H,CH 2 ),2.60(t,J=6.8Hz,2H,CH 2 ),3.72(s,3H,NCH 3 ), 4.15(t, J=6.8Hz, 2H, CH 2 ), 4.23(t, J=6.8Hz, 2H, CH 2 ),4.33(s,2H,CH 2 ), 4.47(t, J=6.8Hz, 2H, CH 2 ),6.78(d,J=8.8Hz,2H,ArH),6.83(d,J=8.8Hz,1H,ArH),7.18(m,2H,ArH),7.44(d,J=8.8Hz,2H, ArH), 7.423 (d, J=8.8Hz, 2H, ArH), 7.57 (m, 1H, ArH), 8.33 (m, 1H, ArH), 8.92 (br s, 2H, NH 2 ); ESI-MS: m / z 556[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com