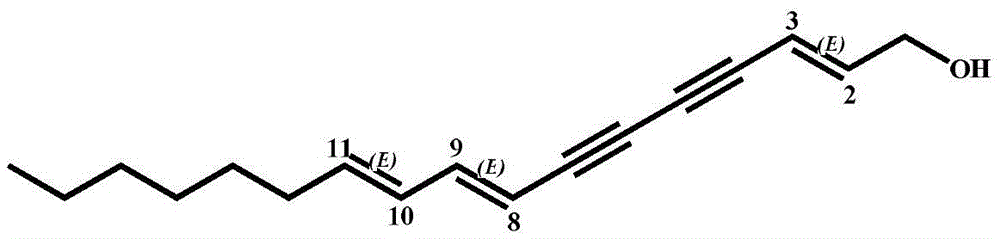
Alkyne compounds as well as preparation method and application thereof
A technology of alkyne compounds and compounds, which is applied in the field of extraction of alkyne compounds and active ingredients of traditional Chinese medicine, and can solve the problems of no activity or toxicity of other compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation of alkyne compound
[0021] (1) Preparation of Bupleurum Petroleum Ether
[0022] 5 kg of Bupleurum medicinal material was soaked in 95% ethanol for 12 hours, refluxed three times for 2 hours each time, the combined extracts were concentrated to obtain extract, and the extract was extracted with petroleum ether to obtain 150 g of Bupleurum petroleum ether fraction.
[0023] (2) Coarse separation of Bupleurum petroleum ether
[0024] Add the above-mentioned Bupleurum petroleum ether fraction sample to a silica gel chromatography column, use petroleum ether-ethyl acetate as the elution system, and sequentially use petroleum ether: ethyl acetate ratio of 100:0-0:100 for gradient elution. Silicone GF 254 Thin-layer chromatography plate and petroleum ether-ethyl acetate are used to develop the system to detect fractions. When the components monitored by thin-layer chromatography change significantly, replace the elution gradient with the next ...
Embodiment 2
[0077] Embodiment 2: Determination of in vitro activity of alkyne compounds
[0078] In vitro 5-HT, NA and DA monoamine transmitter reuptake inhibition tests were carried out on the compounds extracted and separated above.
[0079] 1. Source of experimental materials
[0080] ①Animals
[0081] Male SD rats were purchased from Shanghai Slack Experimental Animal Co., Ltd., body weight: 200-220g, animal certificate number: SCXK (Shanghai) 2012-0002, breeding room level: SPF level animal room for routine feeding.
[0082] ②Reference substance
[0083] Fluoxetine hydrochloride was purchased from the Chemical Division of Shanghai Pharmaceutical Industry Research Institute, desipramine was purchased from Sigma Company, and 6-hydroxydopamine was purchased from Sigma Company.
[0084] 2. Experimental material preparation
[0085] ① Preparation of test samples
[0086] I. Preparation of compounds
[0087] Compounds I, II, III and / or IV were respectively dissolved in 10% DMSO (dime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com