Method for synthesizing leuprorelin by solid phase and liquid phase
A leuprolide and liquid-phase technology, applied in the field of synthesizing leuprolide, can solve the problems of secondary conformation change, low yield of HPLC chromatographic purification product, influence on molecular activity, etc., and achieve stable reaction and high yield , the effect of less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
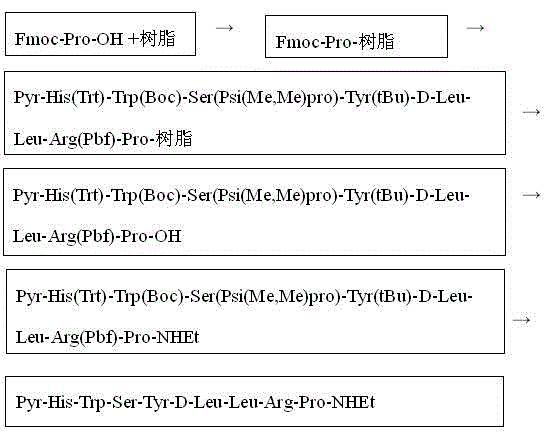
[0036] Embodiment 1: refer to figure 1 , Fmoc-Pro-resin synthesis:
[0037] Put 1000g of 2-CTC resin with a substitution degree of 0.4mmol in the reactor, wash with DCM for 2-3 times, and filter to dry; dissolve 1.2mol of Fmoc-Pro-OH and 2mol of DIEA in DMF, stir and vibrate for 10min, and add to the reactor , mixed with the resin in the reactor, and shaken for 2 hours; after the end, add anhydrous methanol and continue to shake for 30 minutes; after the reaction, filter the reaction solution, and wash the resin alternately with DCM, DMF and anhydrous methanol for 2 to 3 times; The product, Fmoc-Pro-resin, was obtained.
Embodiment 2
[0038] Embodiment 2: refer to figure 1 , Fmoc-Pro-resin synthesis:
[0039] Put 1000 g of 2-CTC resin with a substitution degree of 0.5 mmol into the reactor, wash with DCM for 2 to 3 times, and filter to dry; dissolve 1.5 mol of Fmoc-Pro-OH and 2.5 mol of DIEA in DMF, stir and vibrate for 10 min, and add to the reaction Mix with the resin in the reactor and shake for 2 hours; after the end, add anhydrous methanol and continue to shake for 30 minutes; after the reaction, filter the reaction solution, and wash the resin alternately with DCM, DMF and anhydrous methanol for 2 to 3 times ; Obtain the product namely Fmoc-Pro-resin.
Embodiment 3
[0040] Embodiment 3: refer to figure 1 , the growth of the peptide chain
[0041] Remove the Fmoc protecting group from the Fmoc-Pro-resin twice, each time with 500mL of 20% (V / V) piperidine / DMF for 5 and 15 minutes, and finally wash the resin alternately with DCM, DMF and anhydrous methanol for 2-3 Again, using HBTU / HOBt / DIEA as condensation reagent, repeat the two-step reaction of condensation and deprotection according to the sequence of amino acids in leuprolide from carboxy-terminus to amino-terminus, and assemble Fmoc-Arg(Pbf)-OH sequentially at room temperature , Fmoc-Leu-OH, Fmoc-D-Leu-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Trp(Boc)-Ser(Psi(Me,Me)pro)-OH, Fmoc-His(Trt) -OH, H-Pyr-OH amino acid excess multiple is 1.5 times. During the condensation process, the qualitative color development (chloranil method) was used to detect the progress of the reaction and the Fmoc absorbance method was used to quantitatively detect the condensation efficiency.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com