Nateglinide tablet and method for preparing nateglinide tablet through direct compression method
A technology of glinide tablets and nateglinide, applied in the field of medicine, can solve the problems of splinter, sticking, hemp and the like, and achieve the effect of clean production process and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
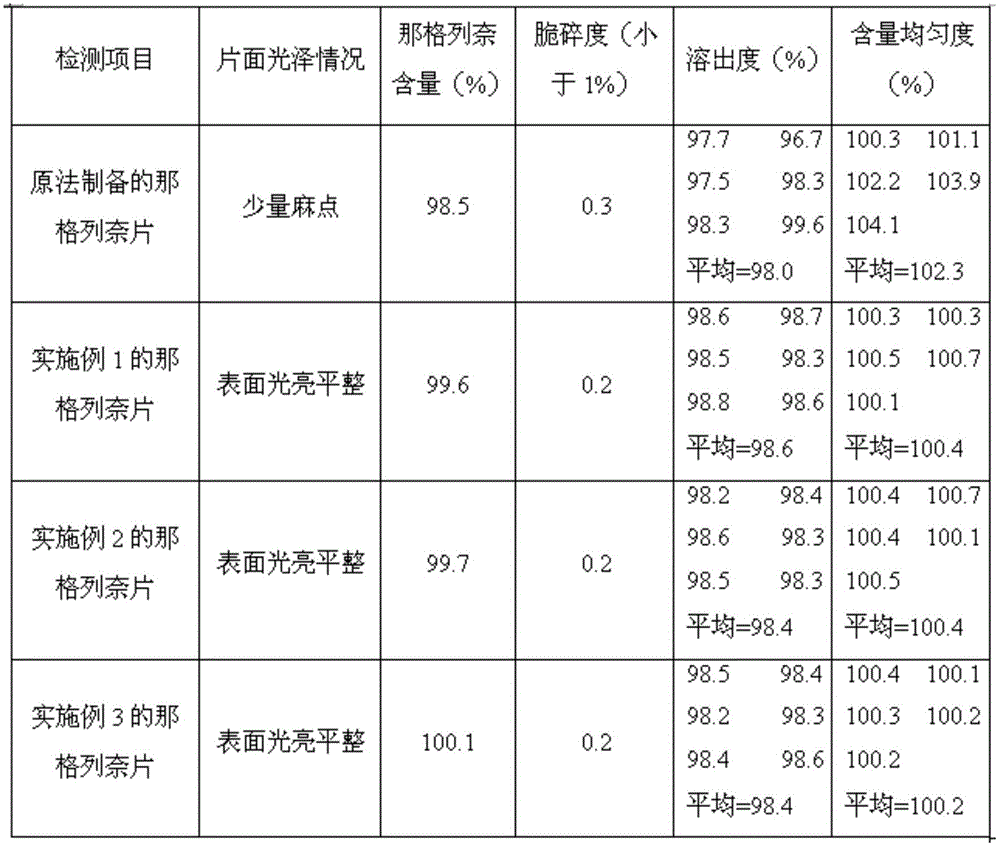
Embodiment 1
[0025] Each 1000 tablets of nateglinide contains:
[0026] Nateglinide 30g;
[0028] Pregelatinized starch 20g;
[0029] Carboxymethyl starch sodium 10g;
[0030] Hypromellose 25g;
[0032] Micronized silica gel 3g.
[0033] Its preparation method comprises the following steps:
[0034] (1) Weighing of raw materials: Weigh 30g of nateglinide, 25g of anhydrous lactose, 20g of pregelatinized starch, 25g of hydroxypropyl cellulose, 10g of sodium carboxymethyl starch, 3g of micropowdered silica gel and stearin in a clean environment of grade D Magnesium acid 1g;
[0035] (2) Sieving: all the raw materials taken by weighing are passed through a 40 mesh sieve respectively, for subsequent use;
[0036] (3) Mixing: add the sieved nateglinide, anhydrous lactose, pregelatinized starch, hypromellose, sodium starch glycolate and micropowder silica gel into the mixer, and mix thoroughly;
[0037] (4) Total blending: i...
Embodiment 2
[0043] Each 1000 tablets of nateglinide contains:
[0044] Nateglinide 30g;
[0045] Anhydrous lactose 25g;
[0046] Microcrystalline cellulose 20g;
[0047] Carboxymethyl starch sodium 20g;
[0048] Hypromellose 15g;
[0050] Micronized silica gel 3g.
[0051] Its preparation method comprises the following steps:
[0052] (1) Weighing of raw materials: Weigh 30g of nateglinide, 25g of anhydrous lactose, 20g of microcrystalline cellulose, 15g of hydroxypropyl cellulose, 20g of sodium carboxymethyl starch, 3g of micropowder silica gel and stearin in a clean environment of grade D Magnesium acid 1g;
[0053] (2) Sieving: all the raw materials taken by weighing are passed through a 40 mesh sieve respectively, for subsequent use;
[0054] (3) Mixing: add the sieved nateglinide, anhydrous lactose, pregelatinized starch, hypromellose, sodium starch glycolate and micropowder silica gel into the mixer, and mix thoroughly;
[0055] (4) Total ble...
Embodiment 3
[0061] Each 1000 tablets of nateglinide contains:
[0062] Nateglinide 30g;
[0063] Anhydrous lactose 25g;
[0064] Microcrystalline cellulose 10g;
[0065] Pregelatinized starch 10g;
[0066] Povidone K905g;
[0067] Carboxymethyl starch sodium 25g;
[0068] Hypromellose 10g;
[0069] Magnesium stearate 1g;
[0070] Micronized silica gel 3g.
[0071] Its preparation method comprises the following steps:
[0072] (1) Weighing of raw materials: Weigh 30g of nateglinide, 25g of anhydrous lactose, 10g of microcrystalline cellulose, 10g of pregelatinized starch, 5g of povidone K905g, 10g of hydroxypropyl cellulose, Sodium starch glycolate 25g, micropowder silica gel 3g and magnesium stearate 1g;
[0073] (2) Sieving: all the raw materials taken by weighing are passed through a 40 mesh sieve respectively, for subsequent use;
[0074] (3) Mixing: add the sieved nateglinide, anhydrous lactose, pregelatinized starch, hypromellose, sodium starch glycolate and micropowder sil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com