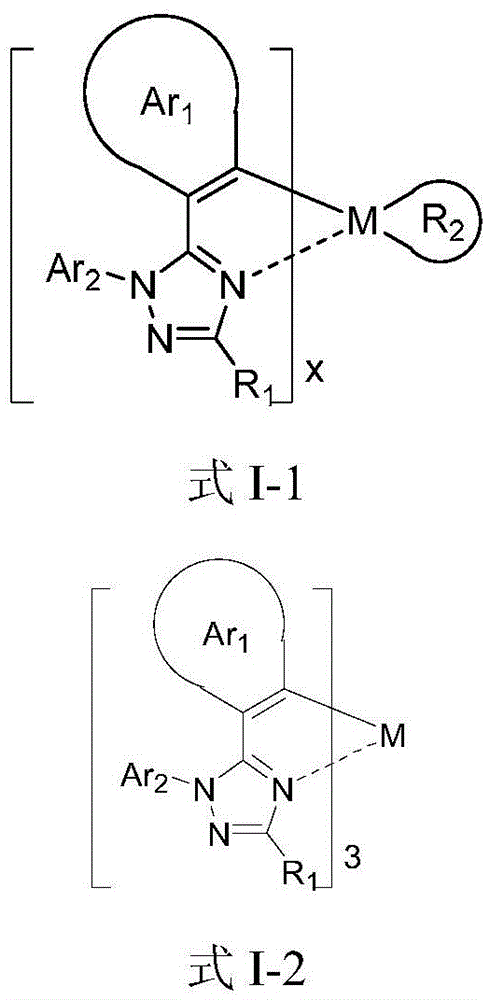
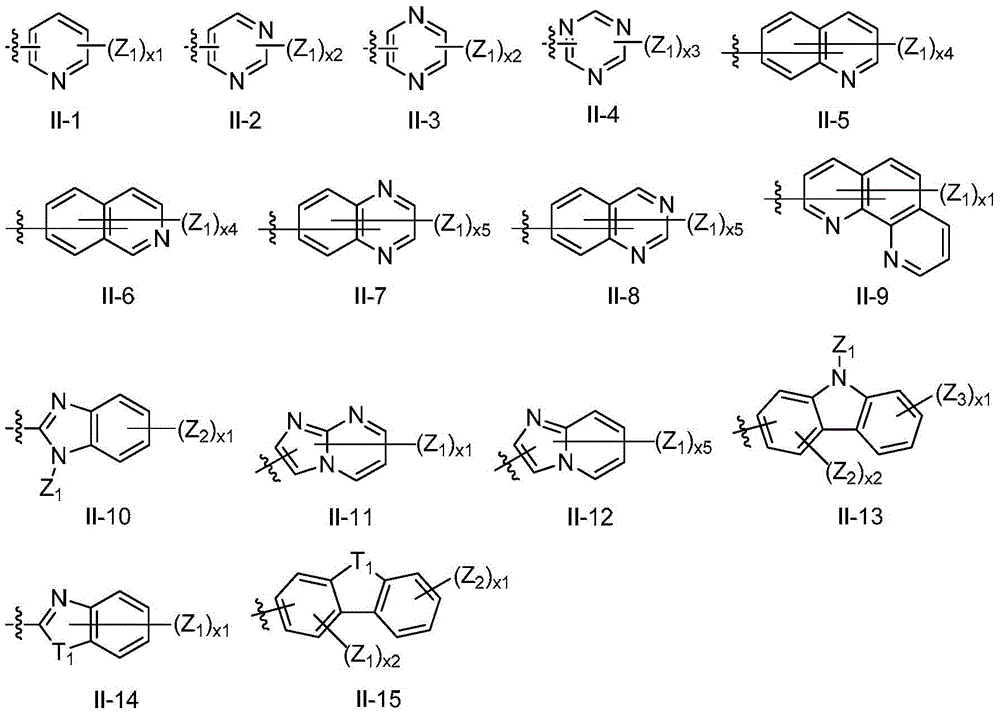
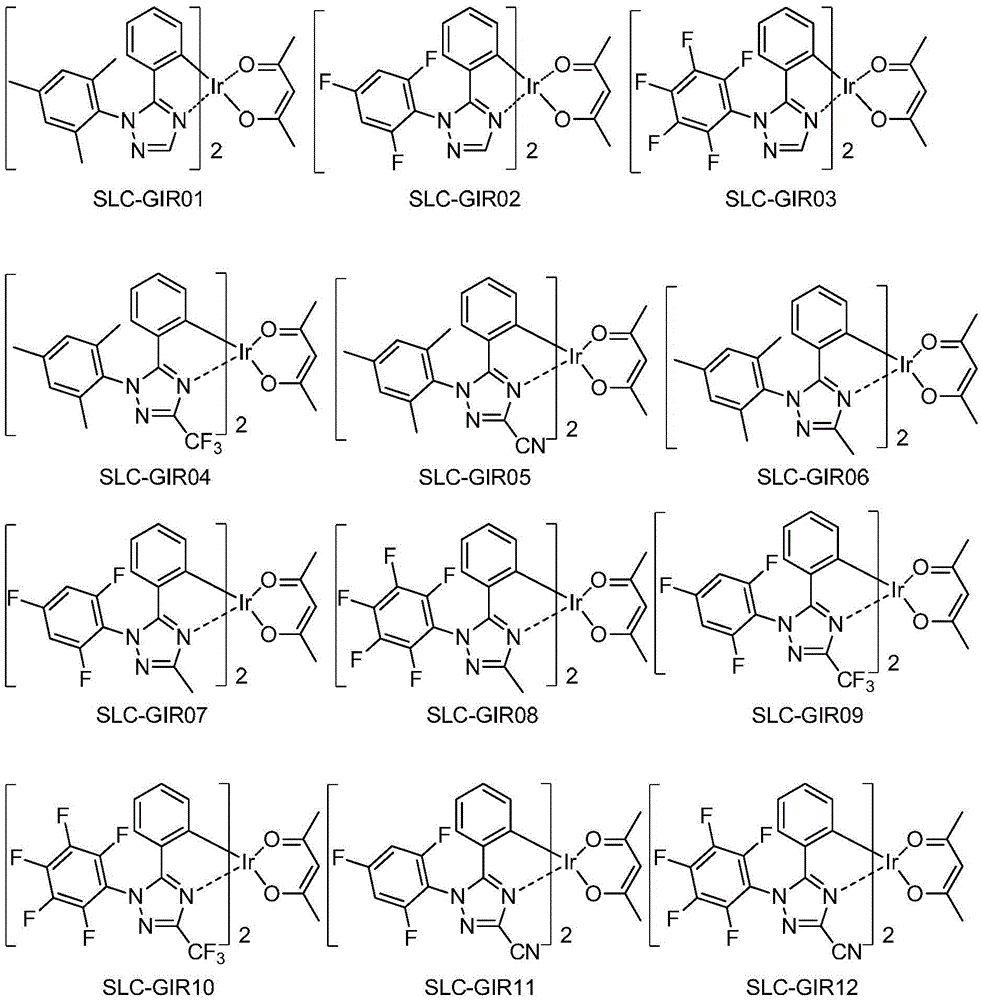
Series of deep blue metal iridium phosphorescence OLED materials
A C6-C60, I-1 technology, applied in the field of phosphorescent OLED materials, can solve the problem that blue metal iridium phosphorescent complexes cannot simultaneously satisfy high color purity luminous efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The preparation of embodiment 1 compound SLC-GIR16
[0071]
[0072] The first step: preparation of compound G-1
[0073]
[0074] 20g of 3,4,5-trifluorobenzamide and 200ml of DMF-DMA, heated to reflux and stirred for 10 hours, concentrated under reduced pressure to dryness, added 150ml of petroleum ether to the residue, stirred and dispersed, and suction filtered to obtain 25g Compound G-1, yellow solid, yield 96%.
[0075] The second step: the preparation of compound G-2
[0076]
[0077] Mix 20g of compound G-1 obtained in the previous step with 13g of 2,4,6-trimethylphenylhydrazine, add 100ml of 1,4-dioxane and 100ml of glacial acetic acid, and heat up to Stir the reaction at 90°C for 12 hours, cool to room temperature, pour the reaction solution into 500ml of ice water, extract the aqueous phase with ethyl acetate, dry the organic phase, filter, concentrate the filtrate to dryness under reduced pressure, and separate and purify the residue with a silica ...
Embodiment 2
[0089] The preparation of embodiment 2 compound SLC-GIR85
[0090]
[0091] 2.0g of SLC-GIR16 and 1.4g of compound G-2 prepared in Example 1 were dispersed with 50ml of glycerin, heated to 180°C under nitrogen protection, stirred for 8 hours, cooled to room temperature, and the reaction solution Poured into 200ml of 1N dilute hydrochloric acid, filtered with suction, washed the filter cake with water, separated and purified the obtained solid with a silica gel column to obtain 1.6g of SLC-GIR85, a yellow solid, with a yield of 64.8%.
[0092] Experimental data:
[0093] (1) 1 HNMR (δ, CDCl 3 ): 1.83(6H,s), 2.12(3H,s), 6.67~6.72(2H,m), 7.09~7.14(1H,m), 8.18(1H,s). LC-MS: 1142.4[M+1] confirms that the substance obtained by the reaction is indeed the compound SLC-GIR85;
[0094] (2) Glass transition temperature (DSC): \;
[0095] (3) UV maximum absorption wavelength (DCM): 295nm, 305nm;
[0096] (4) Phosphorescence emission wavelength (DCM): 470 nm.
Embodiment 3
[0097] The preparation of embodiment 3 compound SLC-GIR62
[0098]
[0099] The first step: preparation of compound G-1
[0100]
[0101]Referring to the synthesis method in the first step of Example 1, replace 3,4,5-trifluorobenzamide in Example 1 with 2,3,4,5-tetrafluorobenzamide to prepare G-1, a yellow solid , yield 86%.
[0102] The second step: the preparation of compound G-2
[0103]
[0104] Referring to the synthesis method in the second step of Example 1, the G-1 obtained in the previous step and 2,4,6-trimethylphenylhydrazine were condensed and ring-closed to obtain G-2, a yellow solid, with a yield of 76%.
[0105] The third step: preparation of compound G-3
[0106]
[0107] With reference to the synthetic method of the third step of Example 1, the G-2 and IrCl obtained in the previous step 3 ·3H 2 O undergoes a coordination reaction to obtain G-3, a yellow solid, with a yield of 82%.
[0108] Step 4: Preparation of Compound SLC-GIR62
[0109] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com