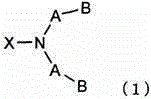
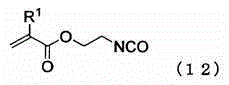
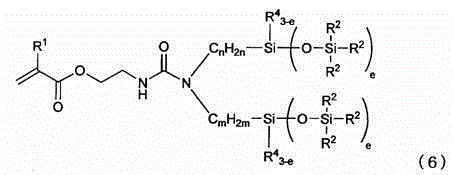
Silicone compound having a radical-polymerizable group and a method for the preparation thereof
A technology of siloxane compounds and polymerizable groups, which is applied in the fields of compounds of Group 4/14 elements of the periodic table, chemical instruments and methods, and medical preparations containing active ingredients, etc. Good handling properties, excellent oxygen permeability, and good reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Add 0.1 mol of siloxane-containing amine of the following formula (I), 200 ml of hexane, and 170 g of 10% sodium carbonate water to a flask equipped with a thermometer, a dropping funnel, and a nitrogen gas introduction tube, and stir at 5° C. 1.2 mol of acryloyl chloride was added dropwise.
[0112] [chem 21]
[0113]
[0114] After the dropwise addition, stirring was continued at room temperature for 5 hours, and then the organic layer was washed with pure water. Next, 0.008 g of p-methoxyphenol (polymerization inhibitor) was added to the washed solution, and the volatile components were distilled off under reduced pressure (60°C, 5 torr) to obtain a light yellow transparent liquid at room temperature (25°C). product. use 1 As a result of identification by H-NMR measurement, it was a compound represented by the following formula (II). The yield was 96.9%.
[0115] [chem 22]
[0116]
[0117] Shown below 1 H-NMR spectrum.
[0118] 1 H-NMR (400MHz, CDCl 3 ...
Embodiment 2
[0120] The method of Example 1 was repeated except that the compound represented by the following formula (III) was used instead of the compound represented by the above formula (I), and a light yellow transparent liquid product was obtained at room temperature (25° C.).
[0121] [chem 23]
[0122]
[0123] use 1 As a result of identification by H-NMR measurement, it was a siloxane compound represented by the following formula (IV). The yield was 97.3%.
[0124] [chem 24]
[0125]
[0126] Shown below 1 H-NMR spectrum.
[0127] 1 H-NMR (400MHz, CDCl 3 ): δ0.00(s,6H), 0.07(s,36H), 0.34~0.48(m,4H), 1.49~1.67(m,4H), 3.18~3.39(m,4H), 5.62(dd,1H ), 6.32 (ddd, 1H), 6.53 (dd, 1H).
Embodiment 3
[0129] Except for using 2-acryloyloxyethyl isocyanate instead of acryloyl chloride, the method of Example 1 was repeated to obtain a pale yellow transparent liquid product at room temperature (25° C.). use 1 As a result of H-NMR measurement and identification, it was a siloxane compound represented by the following formula (V). The yield was 95.2%.
[0130] [chem 25]
[0131]
[0132] Shown below 1 H-NMR spectrum.
[0133] 1 H-NMR (400MHz, CDCl 3 ): δ0.07(s,54H), 0.34~0.45(m,4H), 1.49~1.68(m,4H), 3.08~3.20(m,4H), 3.49~3.59(m,2H), 4.18~4.28 (m, 2H), 4.72 (br, 1H), 5.82 (dd, 1H), 6.15 (ddd, 1H), 6.39 (dd, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com