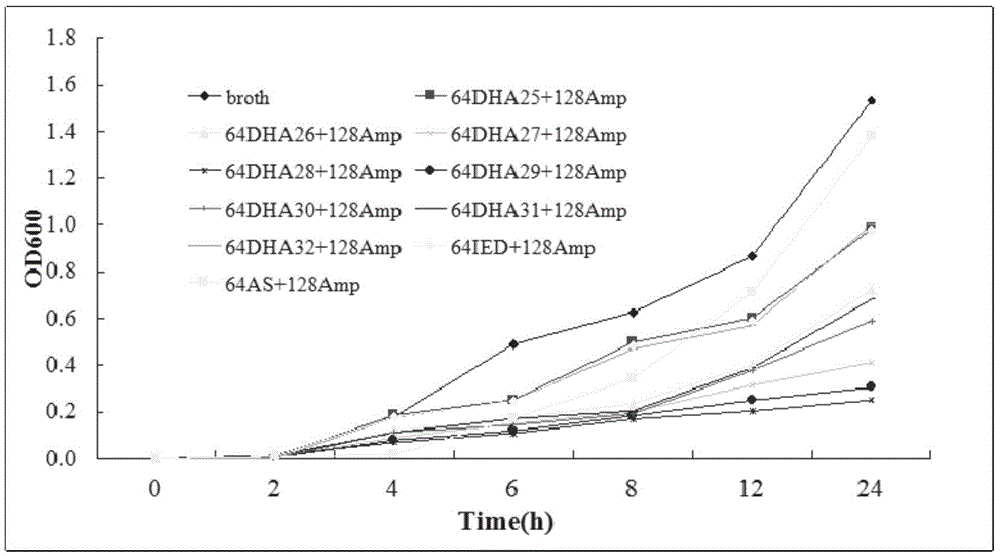
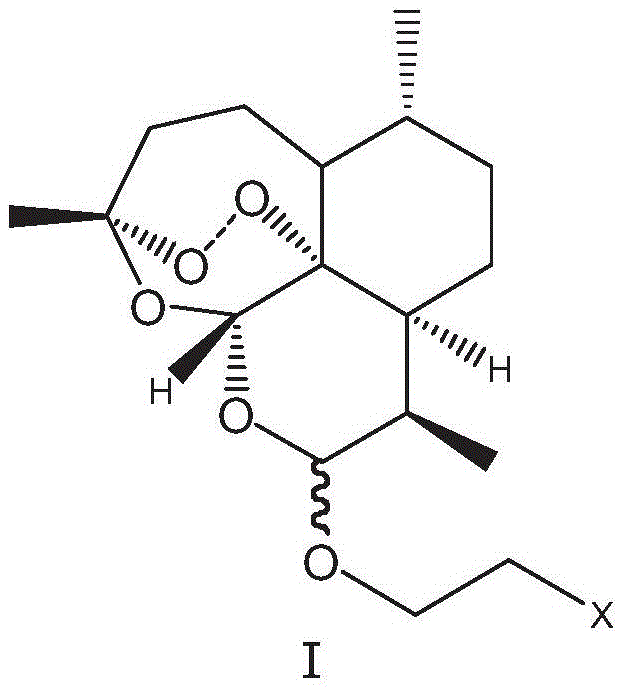
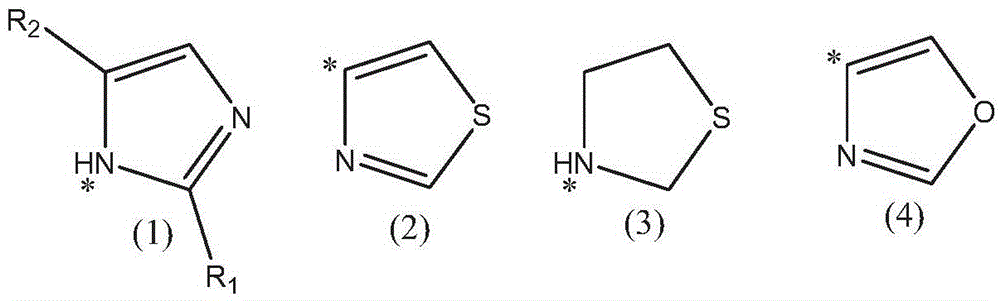
Nitrogen-heterocycle-containing artemisinin derivative and preparation method thereof
A technology of artemisinin derivatives and nitrogen heterocycles, applied in the field of medicine, can solve the problems of increased sensitivity and weak anti-Escherichia coli activity, and achieve the effects of enhanced sensitivity and strong synergistic antibacterial effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 112
[0046] Example 112 Synthesis of β-(2-bromoethoxy) dihydroartemisinin (B)
[0047]
[0048] Add 11.4g (40mmol) of dihydroartemisinin, 2.9ml (40mmol) of 2-bromoethanol and 250ml of THF into a 500ml round bottom flask, add 8ml of BF3.Et2O under the protection of argon, and place it in an ice bath to stir for 6h . The reaction progress was monitored by TLC (EtOAc:PE=1:4). After the reaction was completed, a saturated NaHCO3 solution was added. After separation, the organic layer was collected, the aqueous layer was separated by extraction with EtOAc (60ml×2), and the organic layers were combined. The organic layer was washed with 50 ml of saturated saline solution, then dried over anhydrous MgSO4, and the solvent was distilled off under reduced pressure to obtain a crude product. The crude product was recrystallized in a mixed solvent of petroleum ether and ethyl acetate, filtered and vacuum-dried to obtain 13.1 g of white crystals with a yield of 83.7%.
[0049] MS (ESI, m...
Embodiment 2
[0050] Example 2 Synthesis of DHA25. The synthetic route of 12β-(2-(4-amino-1H-imidazol-1 base)ethoxy)dihydroartemisinin (named DHA25) is as follows:
[0051]
[0052] 1.17g (3mmol) intermediate, 0.41g (3mmol) K 2 CO 3 , 0.049g (0.3mmol) KI and 0.4g (4mmol) 4-amino-1H-imidazole were added to a 100mL round bottom flask. 50mLCH 3 CN was dissolved, and the mixture was reacted at 50° C. for 36 h; the reaction process was monitored by TLC (EtOAc:PE=1:3). After the reaction was completed, 15 mL of dichloromethane and 20 mL of saturated NaCl solution were added. After separation, the organic layer was collected, and the aqueous layer was separated with dichloromethane (10 mL×2) extract, and then the organic layers were combined. The organic layer was washed with 20 mL of saturated brine solution, then washed with anhydrous Na 2 SO 4 Drying; the solvent was removed by distillation under reduced pressure to obtain a crude product. After passing the crude product through the ...
Embodiment 3
[0054] Example 3 Synthesis of DHA26. The synthetic route of 12β-(2-(4-nitro-1H-imidazol-1 base)ethoxy)dihydroartemisinin (named DHA26) is as follows:
[0055]
[0056] 1.22g (3mmol) intermediate, 0.41g (3mmol) K 2 CO 3 , 0.049g (0.3mmol) KI and 0.45g (4mmol) 4-nitro-1H-imidazole were added to a 100mL round bottom flask. 50mLCH 3 CN was dissolved, and the mixture was reacted at 50° C. for 36 h; the reaction process was monitored by TLC (EtOAc:PE=1:3). After the reaction was completed, 15 mL of dichloromethane and 20 mL of saturated NaCl solution were added. After separation, the organic layer was collected, and the aqueous layer was separated with dichloromethane (10 mL×2) extract, and then the organic layers were combined. The organic layer was washed with 20 mL of saturated brine solution, then washed with anhydrous Na 2 SO 4 Drying; the solvent was removed by distillation under reduced pressure to obtain a crude product. After passing the crude product through the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com