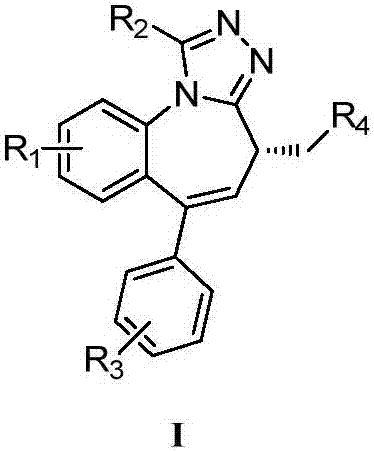
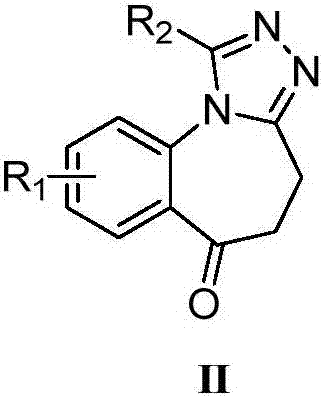
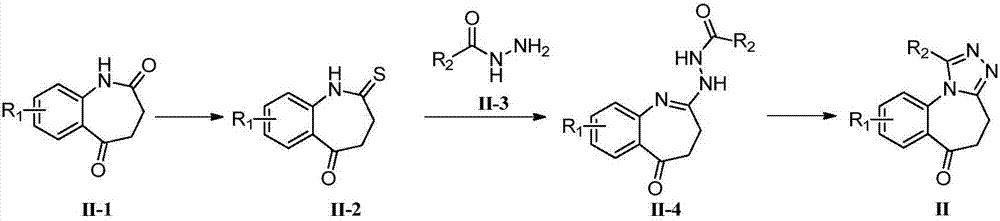
A kind of synthetic method for the key intermediate of synthetic bet protein inhibitor
A synthesis method and reagent technology, which are applied in the field of synthesis of key intermediates used to synthesize BET protein inhibitors, can solve the problems of cumbersome purification, harsh reaction conditions, and low yields, and achieve simple reaction operations, convenient post-processing, The effect of increasing selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of compound 6
[0044]
[0045] Compound 5 (700g, 3.66mol) was dissolved in DMAC (7L), potassium carbonate (1kg, 7.32mol) was added, and methyl iodide (230ml) was slowly added dropwise under ice-water bath conditions. After the dropwise addition, the reaction system was raised to Stir overnight at room temperature. After the reaction was detected by TLC, the reaction solution was filtered and added to EA, washed with water and saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 2.5 kg of a yellow solid crude product, which was directly carried out to the next step without purification.
[0046] 1 H NMR (400MHz, CDCl 3 ):δ7.80-7.75(m,1H),7.55-7.49(m,1H),7.21-7.14(m,2H),3.00-2.94(m,2H),2.77-2.72(m,2H),2.48 (s,3H).
Embodiment 2
[0047] Embodiment 2: the preparation of compound 7
[0048]
[0049] Dissolve compound 6 obtained in the previous step in ethanol (7.5 L), add acetylhydrazide (820 g), and stir at room temperature until the reaction is detected by TLC. A large amount of white solids are precipitated from the reaction system. After the reaction solution is filtered, the filter cake is washed with ethanol successively. , ether, and vacuum-dried to obtain white solid compound 7 (785 g, purity 97%, two-step yield 90%). Without further purification, the next reaction can be carried out directly.
[0050] 1 H-NMR(400MHz,DMSO):δ11.24(s,1H),9.82(s,1H),7.75-7.65(m,1H),7.60-7.45(m,2H),7.15-7.05(m,1H ),2.90-2.80(m,2H),2.75-2.63(m,2H),1.99(s,3H).
Embodiment 3
[0051] Embodiment 3: the preparation of compound 8
[0052]
[0053] Compound 7 obtained in the previous step was dissolved in glacial acetic acid (8 L), and stirred overnight at 100° C., and TLC detected that the reaction of compound 7 was complete. Concentrate the reaction liquid under vacuum conditions, after cooling down to room temperature, add a mixed solvent of methyl tert-butyl ether and petroleum ether, a large amount of light yellow solid precipitates, filter, and recrystallize the obtained filter cake under the condition of isopropanol to obtain the light yellow solid target Compound 8 weighed 553g (purity 99%) after drying the solid, concentrated the crystallization mother liquor, and recrystallized from isopropanol to obtain the light yellow solid target compound 8, weighed 105g (purity 93%) after solid drying , The total yield is 89%.
[0054] 1 H NMR (400MHz, CDCl 3 ):δ7.84-7.80(dd,1H),7.76-7.70(dt,1H),7.59-7.53(dt,1H),7.33-7.28(dd,1H),3.28-3.22(m,2H),3.09...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com