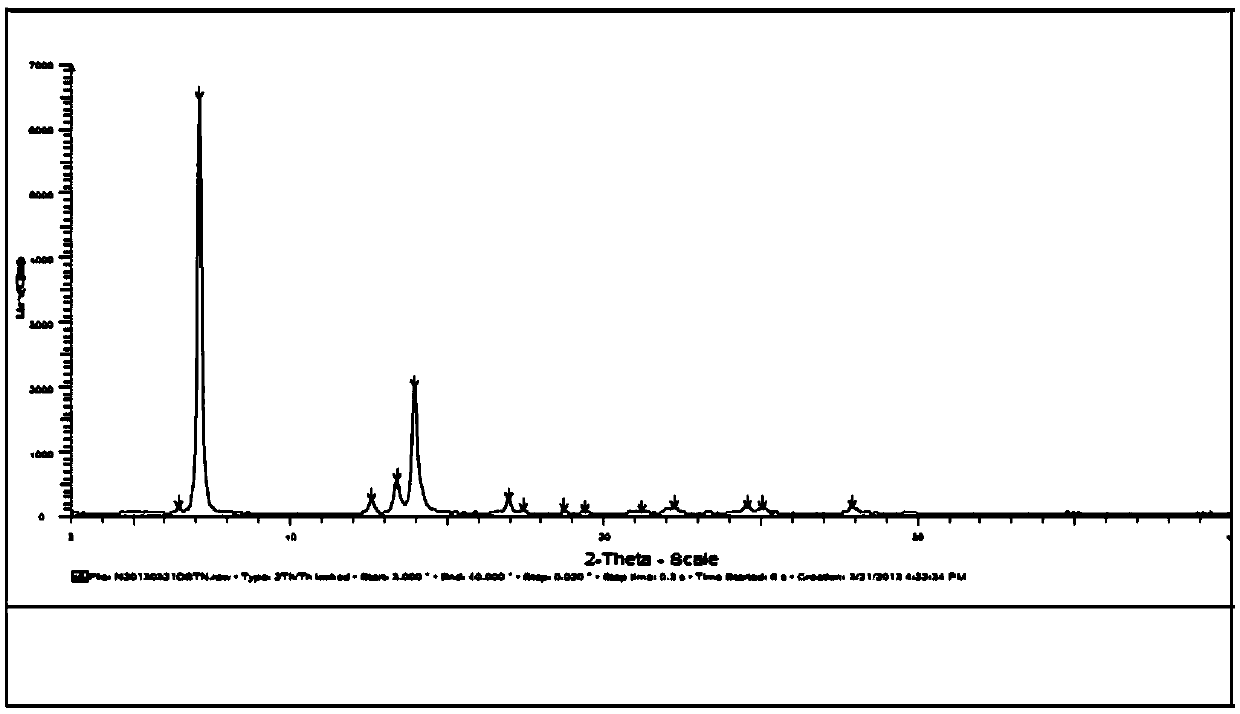
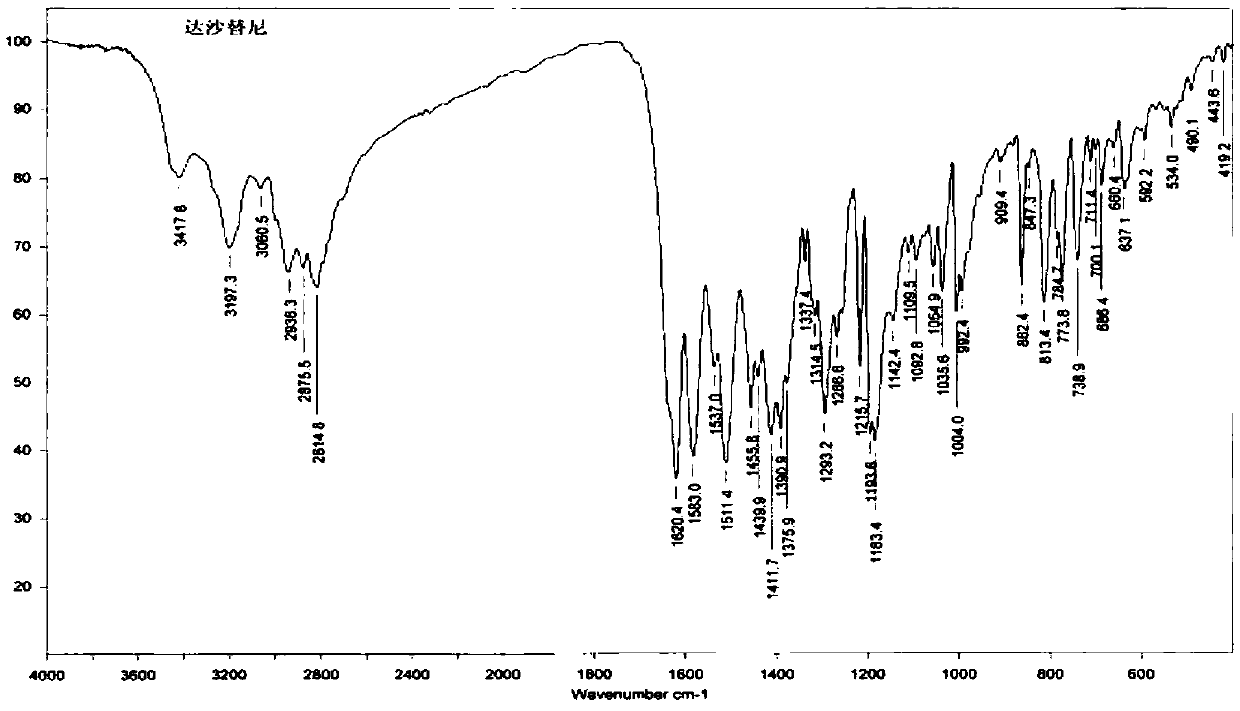
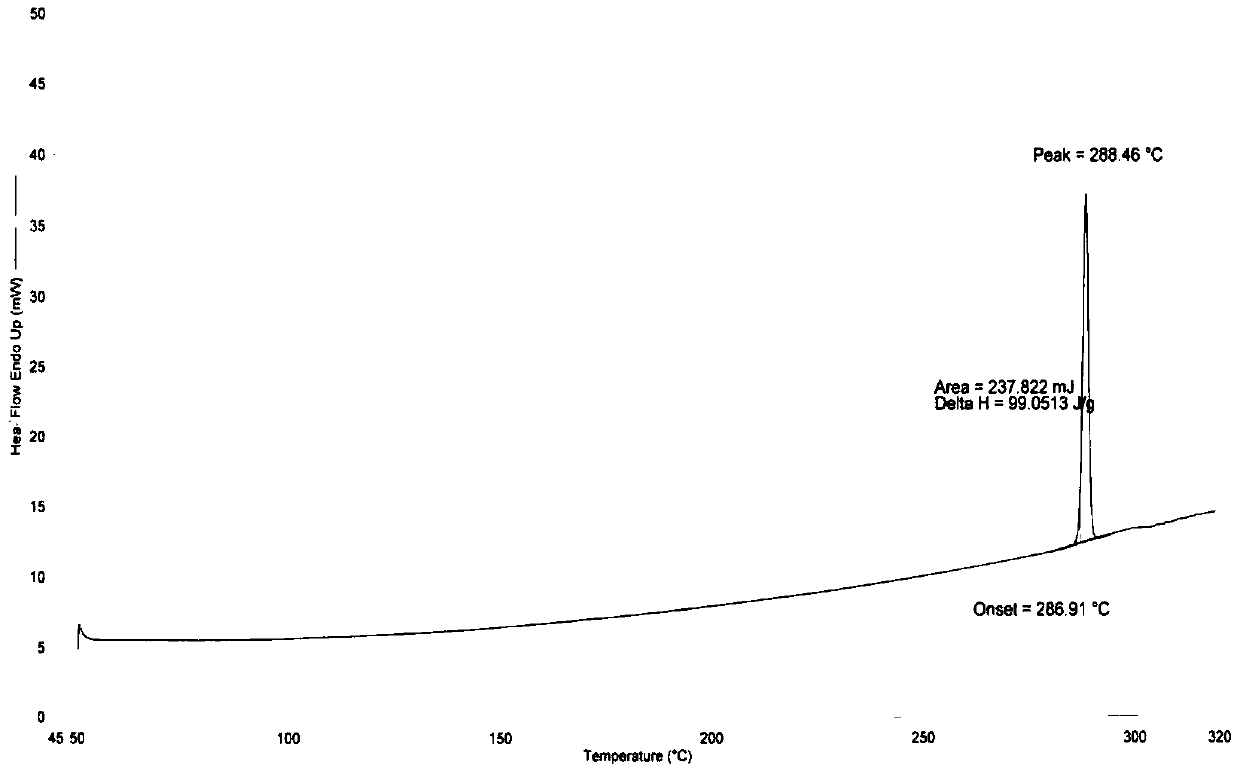
New crystal form substance of Dasatinib anhydrous substance and preparation method thereof
A technology of dasatinib and hydrate, which is applied in the field of new crystal form of dasatinib anhydrate and its preparation, can solve the problem of inability to reduce the product, and achieve industrialization and high reaction selectivity , quality controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Add 12kg of crude dasatinib and 360L of anhydrous methanol into the reactor, stir and heat at room temperature to 65°C, grow crystals for 1 hour, and cool down to room temperature naturally. Suction filtration, washing the filter cake with anhydrous methanol and suction drying, and drying under reduced pressure at 45-55°C and vacuum degree ≤-0.090MPa. Obtained 8.9kg of white new crystalline form of dasatinib anhydrate, with a yield of 74.2% and a product purity of 99.94%.
Embodiment 2
[0056] Add 12kg of crude dasatinib and 240L of anhydrous methanol into the reactor, stir and heat to 40°C, grow crystals for 10 minutes, and cool down to room temperature naturally. Suction filtration, washing the filter cake with anhydrous methanol and suction drying, and drying under reduced pressure at 45-55°C and vacuum degree ≤-0.090MPa. Obtained 7.1 kg of white new crystal form of dasatinib anhydrate, with a yield of 59.2% and a product purity of 99.80%.
Embodiment 3
[0058] Add 12kg of crude dasatinib and 1200L of anhydrous methanol into the reactor, stir and heat at room temperature 25°C, grow crystals for 0.5 hours after complete dissolution, and then cool down to room temperature naturally. Suction filtration, washing the filter cake with anhydrous methanol and suction drying, and drying under reduced pressure at 45-55°C and vacuum degree ≤-0.090MPa. Obtained 8.4kg of white new crystal form of dasatinib anhydrate, with a yield of 70.0% and a product purity of 99.62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com