Binding molecules that bind human complement factor C2 and uses thereof
A technology that combines molecules, complement factors, applied in the field of application of inhibitors of complement factors, application in the treatment of human diseases, diseases or disorders, and can solve the problem of increasing the risk of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Example 1: Generation of Mouse Anti-Human Complement C2 Antibodies
[0133] (a) Production of recombinant human complement C2
[0134] Recombinant human complement protein C2 with an N-terminal his tag was generated by U-ProteinExpress (Utrecht, The Netherlands). Briefly, cDNA encoding human complement protein C2 (GenBank sequence: NM_000063; see SEQ ID NO. 1) was cloned and subsequently expressed in HEK293 cells. Purify C2 by affinity chromatography and use precast gels System (Invitrogen) SDS-PAGE analysis. Proteins were stained with Coomassie brilliant blue.
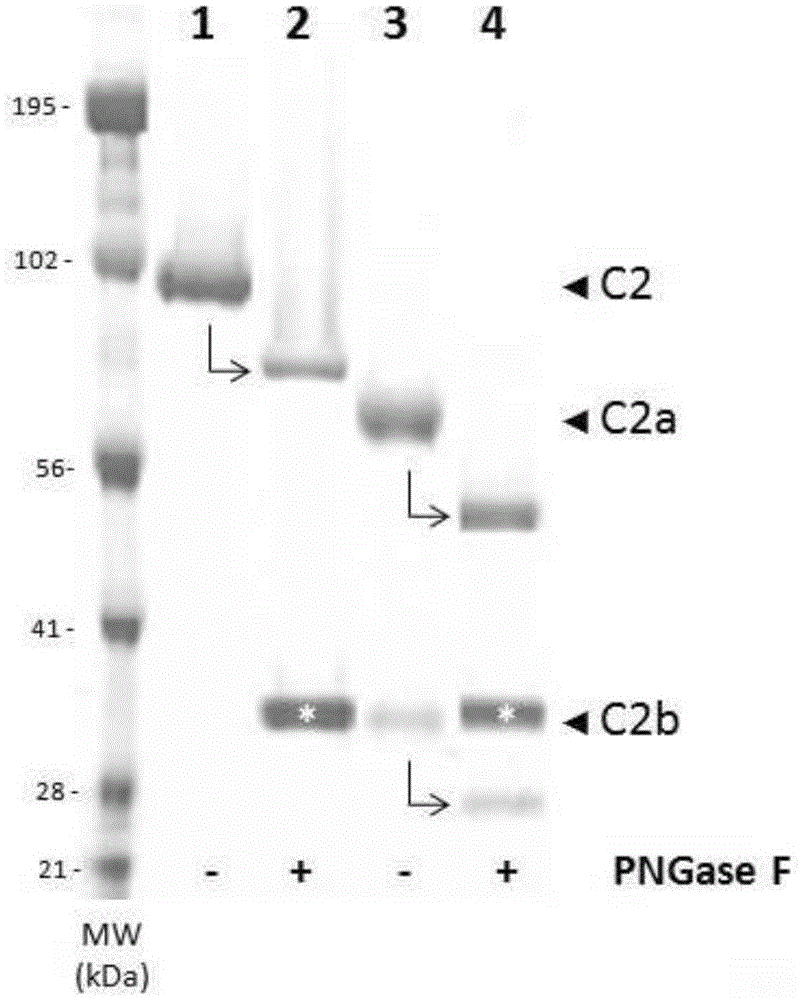
[0135] as in figure 1 As shown in (lane 1 ), the C2 preparation showed >95% purity, ie a band with a molecular weight ≈100 kDa was observed, consistent with the expected molecular weight of glycosylated human C2.
[0136] (b) Biochemical characteristics of recombinant human complement C2
[0137]Generated by incubating C2 (100 μL of a 400 μg / mL solution) with plasma-derived activated C1s (100 μL of a 16 ...
Embodiment 2
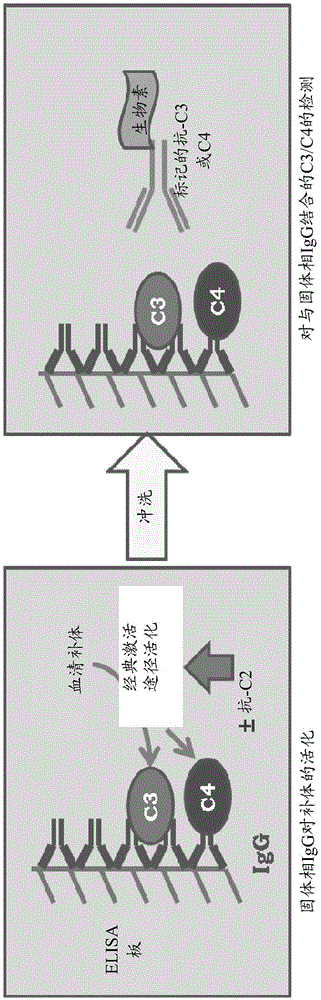
[0144] Example 2: ELISA for Screening the Inhibitory Activity of Anti-C2 Hybridoma Supernatants
[0145] In the ELISA system, the culture supernatants of hybridomas producing anti-C2 antibodies were initially screened for their inhibitory effect on C2 ( figure 2 ). In this ELISA, agglutinated human IgG is coated in microtiter plates (Greiner-Bio-One) and incubated with diluted fresh serum with or without anti-C2 containing hybridoma The supernatant was pre-incubated. Immobilization of C4 and C3 on the plate, indicative of complement activation, was then measured using biotinylated polyclonal affinity purified anti-C3 and anti-C4 antibodies. Streptavidin-HRP was then used to measure the binding of anti-C3 and anti-C4 to the plate, which was visualized using the 3',5'-tetratmethylbenzidine sequence. Briefly, ELISA plates (Greiner-Bio-One) were coated with 100 μl / well of aggregated IgG at 10 μg / ml in PBS overnight at room temperature. In the ELISA, this step and all subseque...
Embodiment 3
[0147] Example 3: Binding characteristics of purified murine anti-C2 antibodies 13, 32, 35, 60 and 5F2.4
[0148] (a) Binding of monoclonal antibodies anti-C2-13, -32, -35, -60 and -5F2.4 to glycosylated recombinant human C2
[0149] Inhibitory and non-inhibitory anti-C2 mAbs were purified using protein G affinity chromatography (GE Healthcare). IsoQuick for isotyping using mouse monoclonal TM Kit (Sigma), heavy and light chains were typed into isotypic species (Table 1).
[0150] Table 1. Antibody classes for some murine mAbs against human C2
[0151]
[0152]
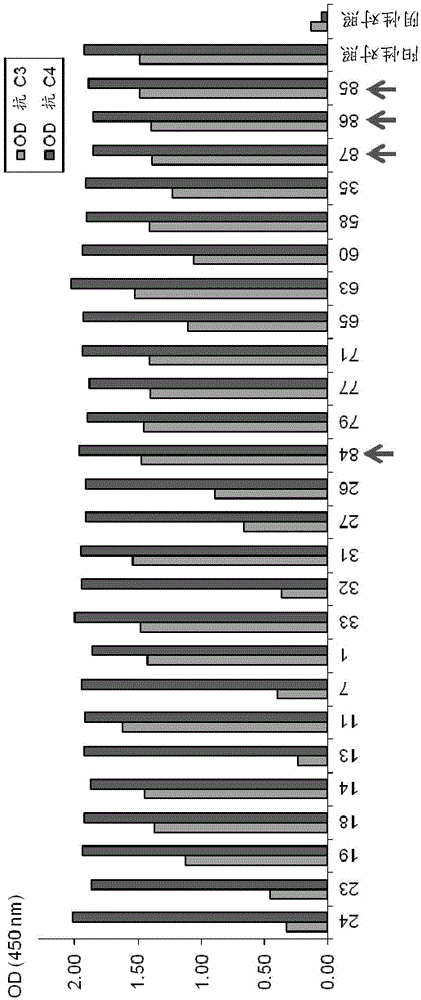
[0153]Using the procedure as described in Example 1(c) above, by assaying for binding to high (200 ng / well) and low (25 ng / well) glycosylated recombinant C2 (U-protein Express), purified The monoclonal antibodies anti-C2-5F2.4, anti-C2-13, anti-C2-32, anti-C2-35 and anti-C2-60 were further characterized.
[0154] as in Figure 4 Antibodies anti-C2-5F2.4, anti-C2-13, anti-C2-32, anti-C2-35 and anti-C2-60 dos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com