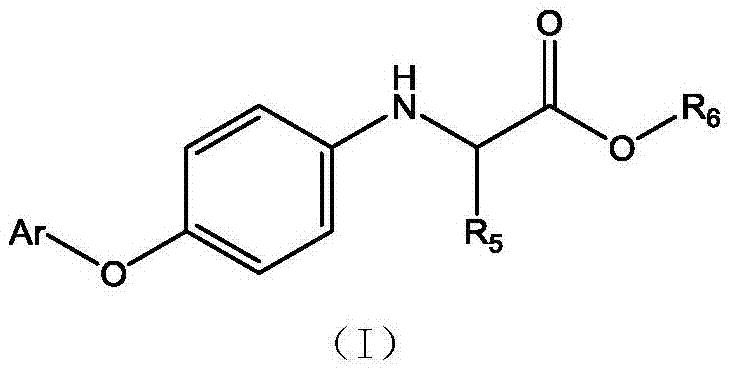
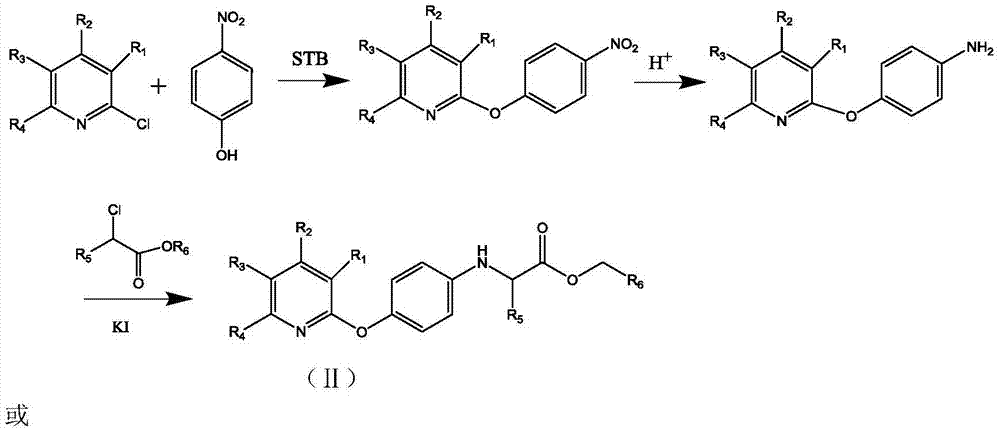
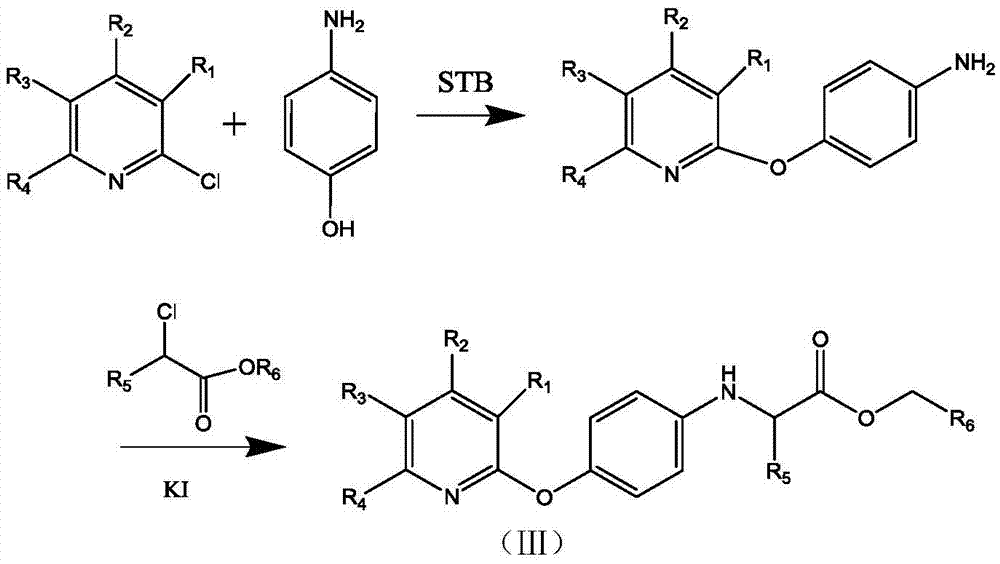
Aryloxy anilino propionic ester compound and application thereof as herbicide
A technology of aryloxyanilinyl propionate and compound, which is applied in the field of preparation of pesticide compounds, can solve the problems of limited application range and unsatisfactory effect on broad-leaved weeds, and achieve the effect that resistance is not easily produced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1,2-[4-(3-chloro-5-trifluoromethyl-2-pyridyloxy) anilino] synthesis of ethyl propionate
[0027] 1. In a 100mL three-necked flask with a nitrogen device, add 40mL of DMF, then add 2.76g (0.02mol) of p-nitrophenol and 1.92g (0.02mol) of sodium tert-butoxide (STB), and stir at room temperature About 2h, then add 4.32g (0.02mol) 2,3-dichloro-5-trifluoromethylpyridine and 2.76g (0.02mol) K 2 CO 3 , continue to stir the reaction at room temperature, use TLC to detect the reaction, after the reaction is over, distill off the DMF solvent under reduced pressure, dissolve it with 50ml chloroform, extract with distilled water, take the chloroform layer, add anhydrous sodium sulfate to dry, stand overnight, filter and concentrate Obtain a dark brown powder; add 120 mL of absolute ethanol, 24 mL of distilled water, 9.6 g of ammonium chloride, and 6.5 g of reduced iron powder to the above dark brown powder, heat to reflux, and detect the reaction by TLC. Wash with disti...
Embodiment 2
[0033] Embodiment 2,2-[4-(5-trifluoromethyl-2-pyridyloxy) anilino] synthetic of ethyl propionate
[0034] 1. In a 100mL three-necked flask with a nitrogen device, add 40mL of DMF, and then add 2.18g (0.02mol) of p-nitrophenol and 1.92g (0.02mol) of sodium tert-butoxide (STB), and stir at room temperature React for about 1h, then add 3.63g (0.02mol) 2-chloro-5-trifluoromethylpyridine and 2.76g (0.02mol) K 2 CO 3 , continue to stir the reaction at room temperature (about 20 ° C); use TLC to detect the reaction, after the reaction is over, distill off the DMF solvent under reduced pressure, dissolve it with 50ml chloroform, extract with distilled water, take the chloroform layer, add anhydrous sodium sulfate to dry, static Leave overnight, filter and concentrate to obtain a dark brown powder; add 120 mL of absolute ethanol, 24 mL of distilled water, 9.6 g of ammonium chloride, and 6.5 g of reduced iron powder to the above dark brown powder, heat to reflux, and detect the reactio...
Embodiment 3
[0040] Embodiment 3,2-[4-(3-trifluoromethyl-2-pyridyloxy) anilino base] the synthesis of ethyl propionate
[0041] 1. In a 100mL three-necked flask with a nitrogen device, add 40mL of DMF, then add 2.18g (0.02mol) of p-aminophenol and 1.92g (0.02mol) of sodium tert-butoxide (STB), and stir the reaction at room temperature About 1h, then add 3.63g (0.02mol) 2-chloro-3-trifluoromethylpyridine and 2.76g (0.02mol) K 2 CO 3 , continue to stir the reaction at room temperature; use TLC to detect the reaction, after the reaction is over, distill off the DMF solvent under reduced pressure, dissolve it with 50ml chloroform, extract with distilled water, take the chloroform layer, add anhydrous sodium sulfate to dry, let stand overnight, filter and concentrate To obtain a dark brown powder, add 120 mL of absolute ethanol, 24 mL of distilled water, 9.6 g of ammonium chloride, and 6.5 g of reduced iron powder to the above dark brown powder, heat to reflux, and detect the reaction by TLC. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com