Targeting tumor neovasculature with modified chimeric antigen receptors
A chimeric antigen receptor and targeting moiety technology, which can be used in anti-tumor drugs, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, microorganisms, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
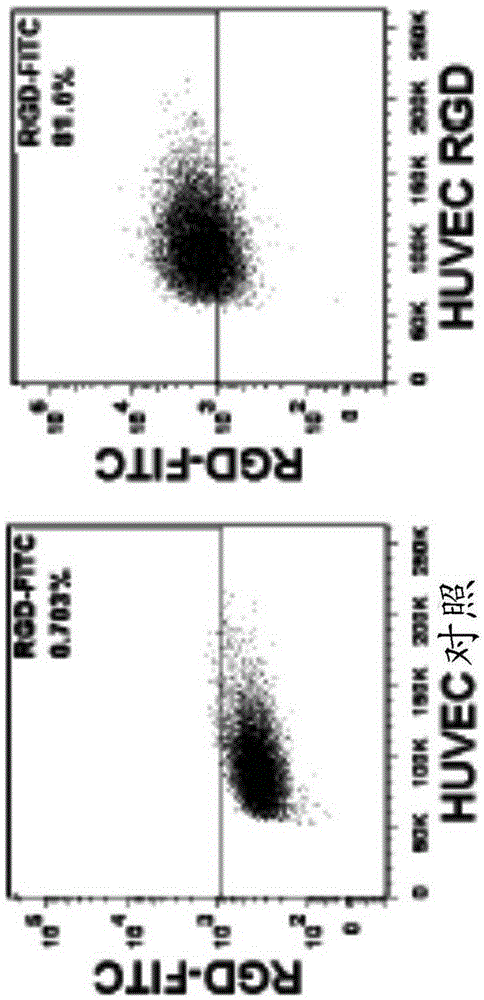
[0055] cell line. Human umbilical vein endothelial cells (HUVEC) and the murine melanoma cell line B16-F0 were obtained from ATCC (Manassas, VA). HUVEC were cultured in Dulbecco's Modified Eagle's Medium (DMEM; Cat. No. 30-2002) formulated by ATCC containing 20% fetal bovine serum (FBS), and B16-F0 cells were grown in a medium containing 100 μg / ml Streptomycin and 100U / ml penicillin in 10% FBSDMEM. B16-GFPluc cells were established in the inventor's laboratory by co-transfecting pIR-eGFP-luc and pCMV-piggyBac plasmids into B16-F0, followed by flow cytometry sorting and single cell cloning as described previously (FuX, et al., Asimple and sensitive method for measuring tumor-specific T cell cytotoxicity. PLoS One 2010; 5: e11867 (2010)).
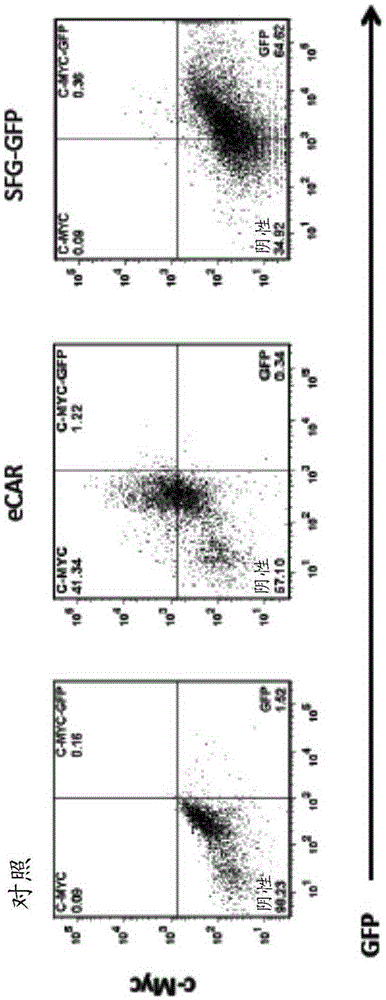
[0056] Retroviral vector construction and preparation. The construction of the retroviral vector is schematically shown in Figure 1Amiddle. The coding sequence for Leu-28-serastatin (MECESGPCCRCKFLKEGTICKRARGDDLDDYCNGKTCDCPRNPHKGPAT; G...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com