Gene diagnosis reagent kit for systemic lupus erythematosus
A lupus erythematosus and genetic diagnosis technology, applied in the field of molecular biology, can solve the problems that diagnosis plays a big role, time-consuming and labor-intensive, and increases consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Gene identification and primer optimization
[0030] According to the previous research, the type I interferon-induced genes related to the diagnosis of SLE were determined to be LY6E, ISG15 and OASL. Through a large number of repeated experiments, combined with the optimization of the template amount and the Tm value of the primers, the primers for amplifying the above three genes were determined. and primers to amplify the housekeeping gene RPLPO.
[0031] For specific primer sequences, see the sequence listing (OASL: SEQ ID NO.1-2; ISG15: SEQ ID NO.3-4; LY6E: SEQ ID NO.5-6; RPLPO: SEQ ID NO.7-8).
[0032] 2. Processing of samples to be tested
[0033] In the morning, 2-3ml of venous whole blood was collected with EDTA venous vacuum disposable blood collection tubes (BD Biosciences, USA) in a resting state on an empty stomach, and then RNA was extracted with Trizol (Invitrogen, USA), and reversed with SuperscriptII reverse transcription kit (Invitrogen, USA). rec...
Embodiment 2
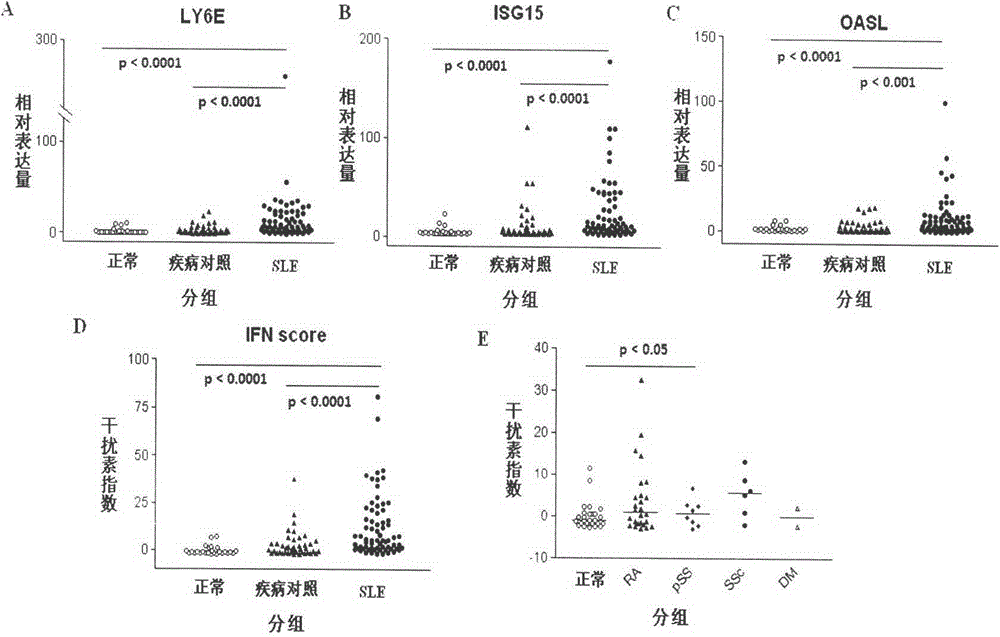
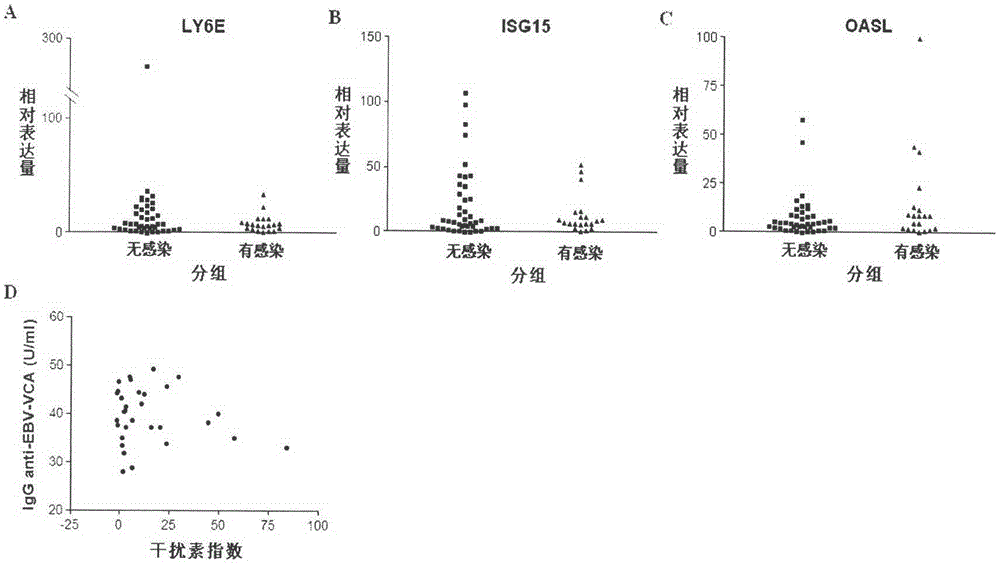
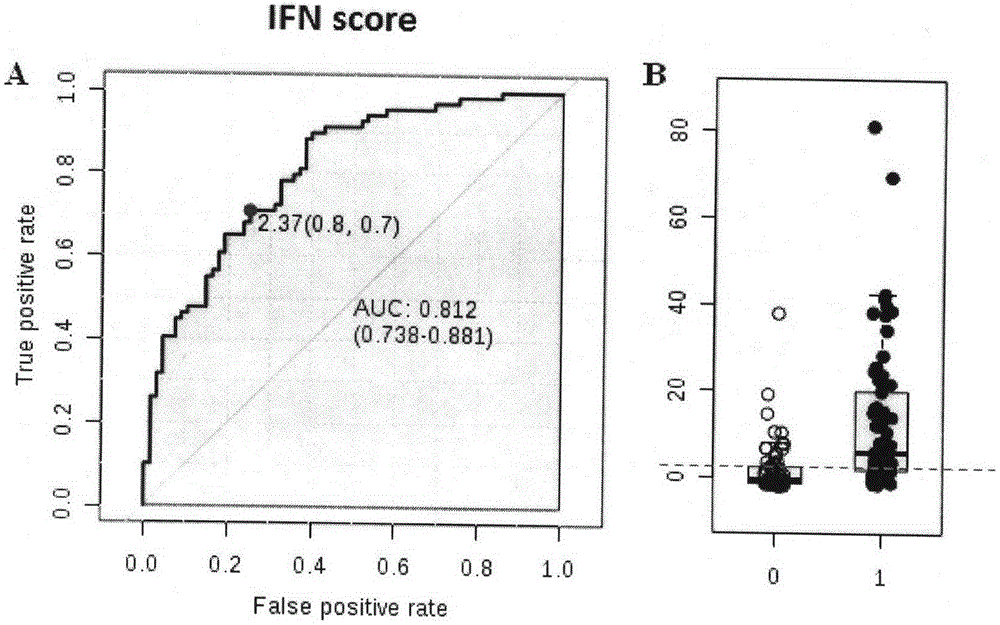
[0041] 1. Comparing the changes of modified interferon index between SLE patients and normal population and patients with other autoimmune diseases
[0042]Blood samples were collected from normal people, SLE patients and patients with other autoimmune diseases, the relative expression values of each gene were obtained according to Example 1, and the expression levels of LY6E, ISG15 and OASL genes in each group were compared. Standardize the level of each gene by (relative gene expression value of the sample to be tested-average relative gene expression value of the normal group) / standard deviation of gene expression in the normal group, and then add the three values corresponding to LY6E, ISG15 and OASL to obtain The modified interferon index was used to compare the differences in the levels of the modified interferon index in each group. Compared with normal people and patients with other autoimmune diseases, the expression levels of LY6E, ISG15, OASL and modified interf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com