Preparation method of biotin intermediate impurity
An intermediate, biotin technology, applied in the direction of magnesium organic compounds, organic chemistry, etc., can solve the problems such as the inability to obtain high-purity transition state hydroxyl compounds, and achieve the effect of simple and easy preparation method and quality control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
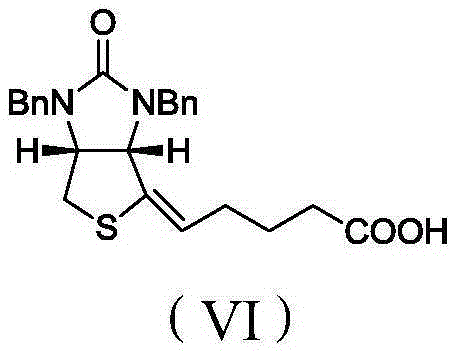
[0048] Biotin intermediate impurity 5-((3aS,4S,6aR)-1,3-dibenzyl-4-hydroxy-2-oxo-1H-thiophene[3,4-d]imidazole-4-yl)pentyl The preparation method of acid, comprises the following steps:
[0049] a) Grignard reaction
[0050] Under the protection of nitrogen, add 5.8g of magnesium chips and 60ml of anhydrous tetrahydrofuran to the reaction flask, heat and reflux for 30min, add 1 iodine particle (0.3g) to the reaction flask, and dropwise add 25g of 1,4-dihydrofuran to the reaction flask Butyl bromide and 125ml tetrahydrofuran were added dropwise and continued to reflux for 3h; a reaction solution containing a Grignard reagent as shown in structural formula (I) was obtained.
[0051] b) Addition reaction
[0052] Add 21.8g of thiolactone ((3aS,6aR)-1,3-dibenzyltetrahydro-1H-thiophene[3,4-d]imidazole-2,4-dione to the reaction flask, then add 200ml of tetrahydrofuran Dissolved, transferred to the dropping funnel, added dropwise to the reaction solution prepared in step a) under n...
Embodiment 2
[0057] Biotin intermediate impurity 5-((3aS,4S,6aR)-1,3-dibenzyl-4-hydroxy-2-oxo-1H-thiophene[3,4-d]imidazole-4-yl)pentyl The preparation method of acid, its difference with embodiment 1 is only in step C) CO 2 The feeding temperature of the mixture is -20°C, the ventilation time is 4h, and the addition amount of other materials, reaction conditions and post-treatment process are consistent with Example 1. Finally, 42.5 g of the light yellow oily liquid of the final product was obtained. The yield was 83%.
[0058] According to the same method as in Example 1, the content of the obtained final product was 98.7% through HPLC detection.
[0059]It can be seen from the above examples that the process of the present invention is especially suitable for synthesis under laboratory conditions, without harsh reaction conditions, and the resulting product has high purity, which can be used for the detection of impurities in the corresponding intermediates of biotin.
experiment example 1
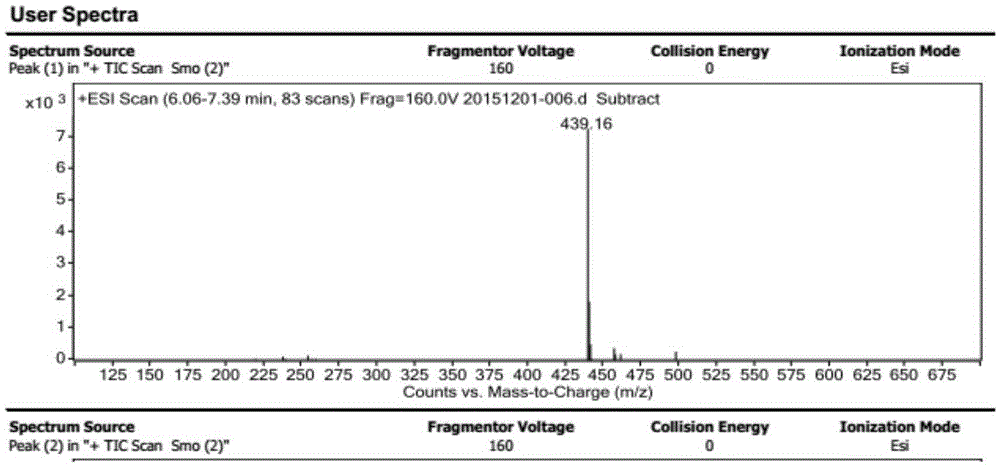
[0061] The mass spectrogram of the final product that embodiment 1 makes is shown in figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com