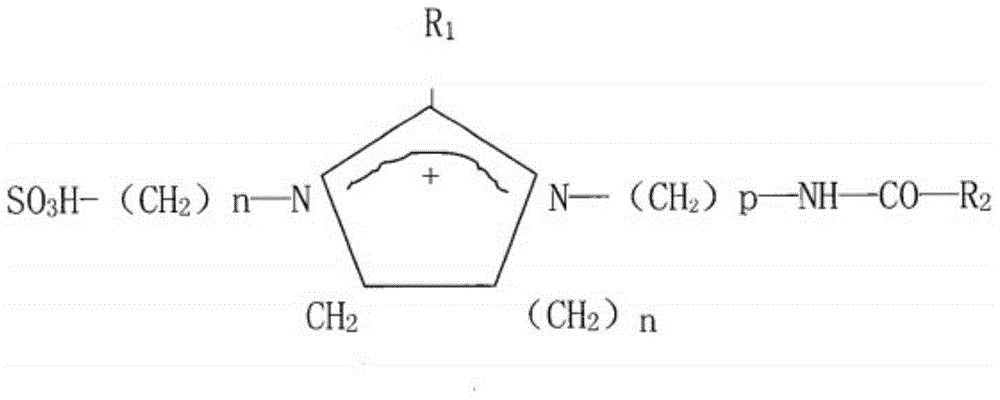
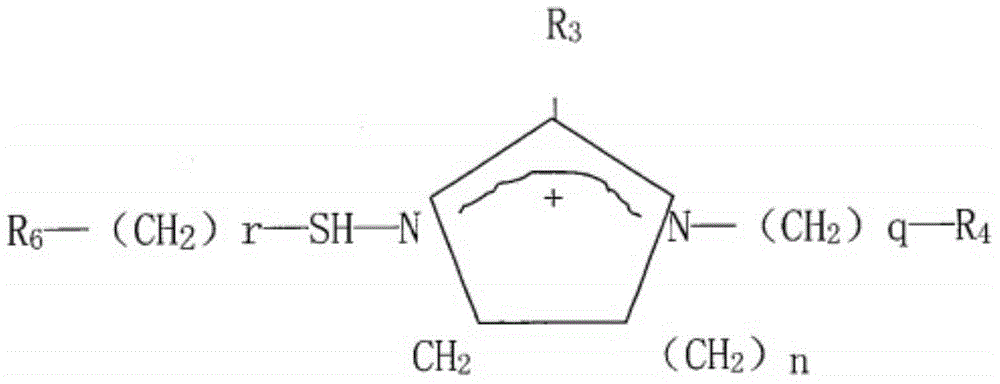
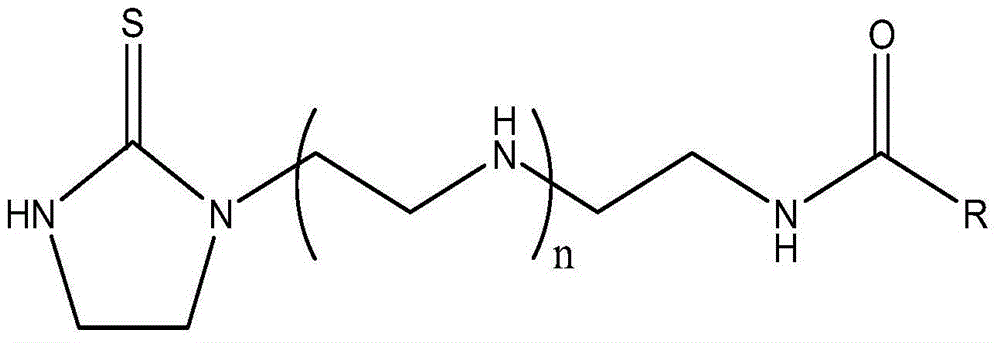
Imidazolyl thiourea derivative, corrosion inhibitor and preparation method thereof
A technology of imidazolyl thiourea and azole thiourea, which is applied in the fields of imidazolyl thiourea derivatives, corrosion inhibitors and their preparation, can solve the problems of inapplicability of corrosion inhibitors and achieve good anti-corrosion effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] In the third aspect, the embodiment of the present invention provides a preparation method of the above imidazolyl thiourea derivative, which comprises: adding thiourea and organic amine into a reactor, and making the thiourea and organic amine be at 150-170°C, preferably at React at a temperature of 160°C for 1.5-3.5h, preferably 2h, while using dilute hydrochloric acid or water to absorb the tail gas generated during the reaction; then add organic acid to the reactor and continue at 150-170°C, preferably at The reaction was carried out at a temperature of 160°C for 1.5-3.5h, preferably 2h, and the product in the reactor was collected to obtain an imidazolyl thiourea derivative; wherein the thiourea, organic amine and organic acid added in the reactor The same amount.
[0041] In the preparation method of the imidazolyl thiourea derivatives, thiourea and organic amines react first to remove a part of the ammonia gas to generate an amino thiourea derivative, and then furth...
Embodiment 1
[0059] In a four-necked flask with a stirrer, a thermometer and a reflux condenser with a branch pipe connected to a water separator, add 1 mole of thiourea and 1 mole of triethylenetetramine in turn, react at 150℃ for 3.5h, and use them at the same time Dilute hydrochloric acid absorbs the tail gas produced during the reaction. Then 1 mole of lauric acid was added to the four-necked flask, reacted at 170°C for 1.5h, and finally collected in the four-necked flask to obtain the final product, that is, the imidazolyl thiourea derivative. The structural formula of the imidazolyl thiourea derivative is:
[0060]
Embodiment 2
[0062] In a four-necked flask with a stirrer, a thermometer, and a reflux condenser with a branch pipe connected to a water separator, add 1 mole of thiourea and 1 mole of tetraethylenepentamine in sequence, and react at 160℃ for 2h while using water Absorb the exhaust gas generated during the reaction. Then add 1 mole of n-octanoic acid to the four-necked flask, react at 160°C for 2h, and finally collect the four-necked flask to obtain the final product, that is, the imidazolyl thiourea derivative. The structural formula of the imidazolyl thiourea derivative is:
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com