Pharmaceutical composition, preparation and uses thereof
A technology of composition and medicine, applied in the field of pharmaceutical composition, preparation and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Synthesis of liposomes as biocompatible nanoparticle n°1
[0079] Preparation of liposomes using the lipid membrane rehydration method:
[0080] a) Lipids were dissolved in chloroform. Chloroform was finally evaporated under nitrogen flow. Rehydration of the lipid film was performed at 50° C. with HEPES 20 mM pH 7.4 and NaCl 140 mM so that the lipid concentration was 5 mM.
[0081] The following lipid compositions were used to prepare charged liposomes: DPPC (dipalmitoylphosphatidylcholine): 86% moles; MPPC (monopalmitoylphosphatidylcholine): 10% moles; DSPE-PEG (di Stearoylphosphatidylethanolamine-[methoxy(polyethylene glycol)-2000]): 4 mole%.
[0082] b) Six thawing cycles were then performed by successively placing the sample in liquid nitrogen and in a water bath regulated at 50°C.
[0083] c) Under controlled temperature and pressure, using a hot barrel extruder (LIPEX TM extruder, NorthernLipids) to calibrate the size of the liposomes. In all case...
Embodiment 2
[0087] Example 2: A method that allows for a dose reduction of at least 10% of a therapeutic compound to be administered in a subject with equivalent efficacy in said subject.
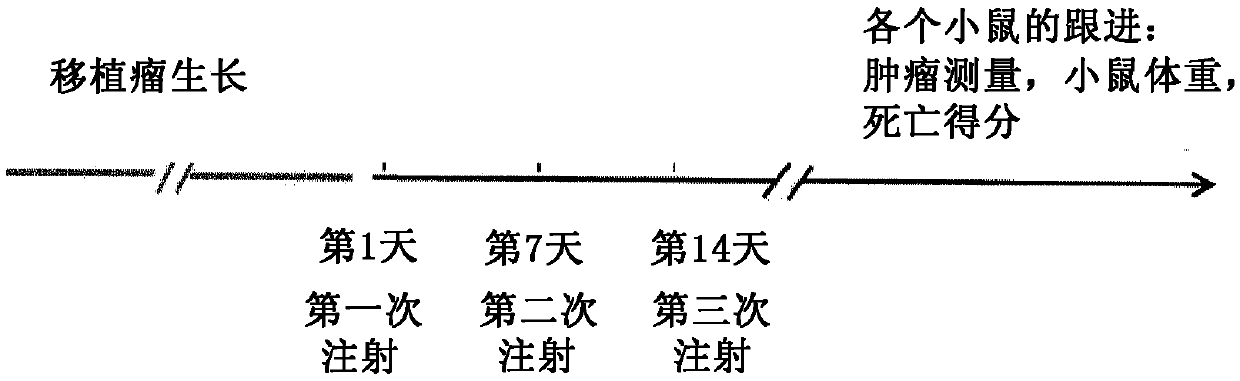
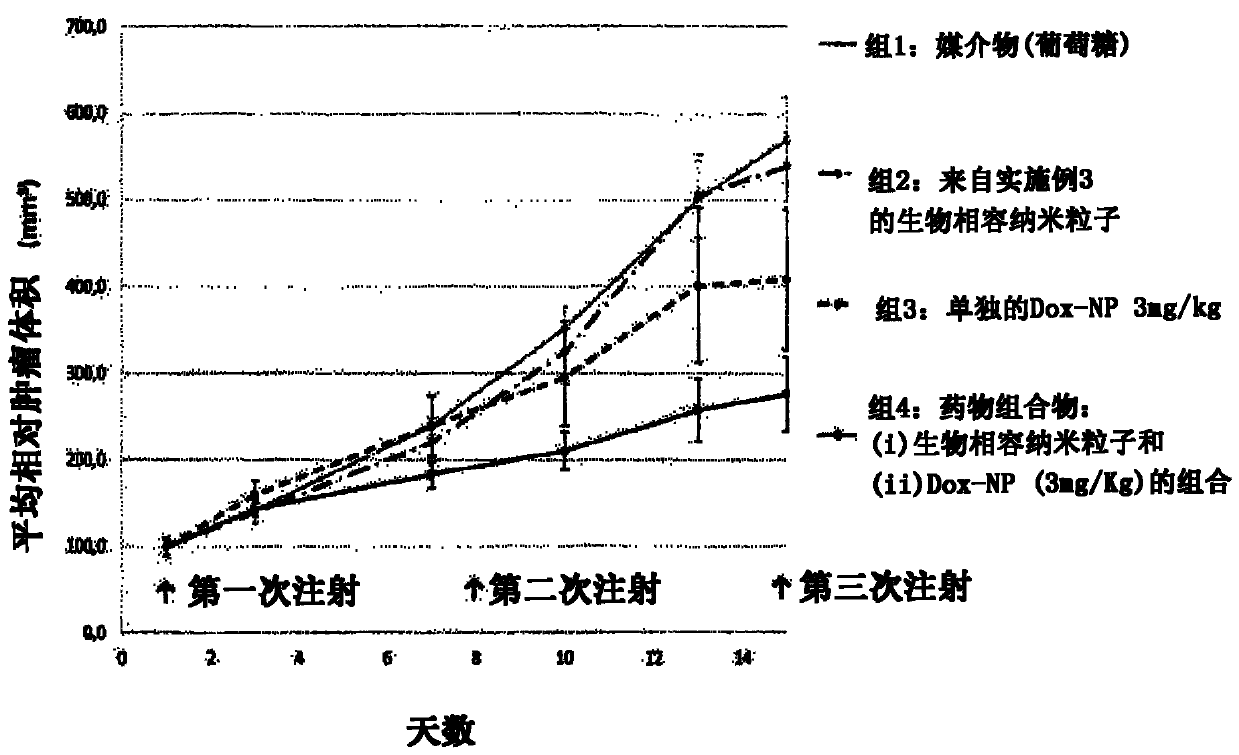
[0088] The pharmaceutical composition according to claim 1 is administered in xenografted nude mice in such a manner that said pharmaceutical composition comprises biocompatible nanoparticles and is capable of generating electrons and / or when exposed to ionizing radiation such as X-rays or high-energy photon-activatable oxide nanoparticles for anti-cancer therapy (used as "the compound" or "drug compound"):
[0089] a) administering biocompatible nanoparticle to each nude mouse (by intravenous injection);
[0090] b) between greater than 5 minutes and 72 hours after step a), administer (by intravenous injection) the therapeutic compound in each mouse of step a) at a dose lower (10%) than the currently used dose;
[0091] c) Measuring the concentration of the therapeutic compound in blood or plasma sam...
Embodiment 3
[0094] Example 3: Synthesis of liposomes as biocompatible nanoparticle n ° 2
[0095] Preparation of liposomes using the lipid membrane rehydration method:
[0096] a) Lipids were dissolved in chloroform. Chloroform was finally evaporated under nitrogen flow. Rehydration of the lipid film was performed at 60° C. with HEPES 20 mM pH 7.4 and NaCl 140 mM so that the lipid concentration was 25 mM.
[0097] The following lipid compositions were used to prepare charged liposomes: DPPC (dipalmitoylphosphatidylcholine) 62% moles; HSPC (hydrogenated soybean phosphatidylcholine) 20% moles; CHOL (cholesterol) 16% moles ; POPS (1-palmitoyl-2-oleoylphosphatidylserine) 1% mole; DSPE-PEG (distearoylphosphatidylethanolamine-[methoxy(polyethylene glycol)-2000]) 1% mole.
[0098] b) Six thawing cycles were then carried out by successively placing the sample in liquid nitrogen and in a water bath regulated at 60°C.
[0099] c) Under controlled temperature and pressure, using a hot barrel ext...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com