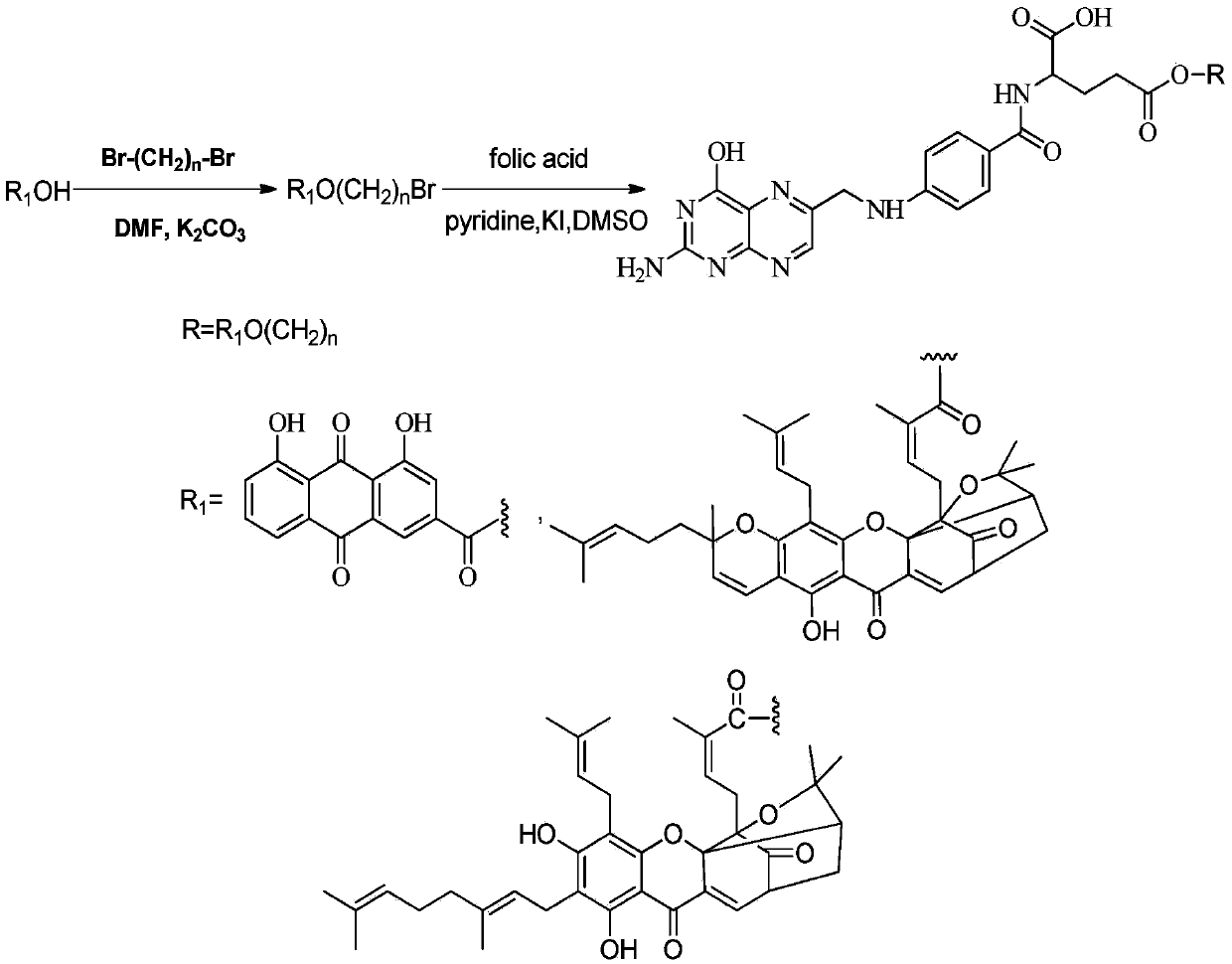
Folic acid compound, and preparation method and pharmaceutical application thereof
A compound, folic acid technology, applied in the field of medicinal chemistry and pharmacotherapeutics, which can solve the problems of lack of drugs, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Synthesis of 6-bromohexyl rhein
[0061] Add rhein (284mg, 1mmol) into a 50mL round-bottomed flask, use THF as a solvent, stir at room temperature for 5 minutes, then add 1,6-dibromohexane (4mmol), triethylamine (4mmol), tetra-n-butyl bromide Ammonium chloride (40.3 mg, 0.25 mmol) was added into the round bottom flask, and the stirring was continued at room temperature. The reaction progress was detected by TLC, and the reaction was complete after 18 h. Concentrate under reduced pressure and separate by silica gel column chromatography. Concentrate under reduced pressure and dry to obtain 404.1 mg of rhein-6-bromohexyl ester as an orange solid, with a yield of 90.6%. m.p.: 131.4-132.6°C.
[0062] Synthesis of Folate-6-Rheinyl Hexyl Ester
[0063] Put 6-bromohexyl rhein (111.5mg, 0.25mmol), folic acid (132.3mg, 0.3mmol), 5 drops of pyridine, KI (49.8mg, 0.3mmol) in a 50mL round bottom flask, add DMSO8mL, 45 The reaction was carried out in the dark at ℃, and the progr...
Embodiment 2
[0065] Synthesis of Gambogic Acid-2-Bromoethyl Ester
[0066] Add gambogic acid (628mg, 1mmol), 1,2-dibromoethane (4mmol), potassium carbonate (138mg, 1mmol), DMF15mL into a 100mL round bottom flask, stir at room temperature, TLC to detect the reaction process, and the reaction is complete after 2h . Add 150 mL of water, extract with ethyl acetate (50 mL×3), combine the organic layers, wash with saturated brine, and dry over anhydrous sodium sulfate. Concentrate under reduced pressure and separate by silica gel column chromatography. Obtained 589.4 mg of gambogic acid-2-bromoethyl ester as an orange solid, with a yield of 80.3%. m.p.: 85.6-86.1°C.
[0067] Synthesis of Folate-2-Gambozoyl Ethyl Ester
[0068] Take folic acid (83.8mg, 0.19mmol), gambogic acid-2-bromoethyl ester (117.4mg, 0.16mmol), 4 drops of pyridine, and 10mL of DMSO in a 100mL round bottom flask, react in the dark at 45°C, and detect the reaction by TLC Process, the reaction was complete after 10.5h. Co...
Embodiment 3
[0070] Synthesis of Neogambogic Acid-3-Bromopropyl Ester
[0071] In a dry 50ml round bottom flask, neogambogic acid (320mg, 0.5mmol), K 2 CO 3 (140mg, 1mmol) and 1,3-dibromopropane (4mmol) were dissolved in 10mL DMF, stirred at room temperature for 1h. Filtrate, add 100 mL of water to the filtrate, extract with ethyl acetate (3×30 mL), combine the organic layers, wash with saturated brine, wash with anhydrous Na 2 SO 4 After drying and filtering, the filtrate was concentrated and separated by silica gel column chromatography. 345mg of bromopropanol neogambogic acid was obtained as a bright yellow oily substance, yield: 92%.
[0072] Synthesis of folate-3-neogamboyl propyl ester
[0073] Take folic acid (132.3mg, 0.3mmol), neogambogic acid-3-bromopropyl ester (187.5mg, 0.25mmol), 7 drops of pyridine, and 10mL of DMSO in a 100mL round bottom flask, react in the dark at 45°C, and detect the reaction by TLC Process, the reaction is complete after 10h. Cool to room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com