Method for synthesis of novel pyrimidine fluorescent opening type optical probe materials and for applying materials in mercury ion detection
An optical probe and pyrimidine-based technology, which is applied in the synthesis field of new pyrimidine-based fluorescent open-type optical probe materials, can solve the problems of poor detection selectivity and anti-interference, fluorescence quenching of probe molecules, etc., and achieve simple detection methods , short time and cheap equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
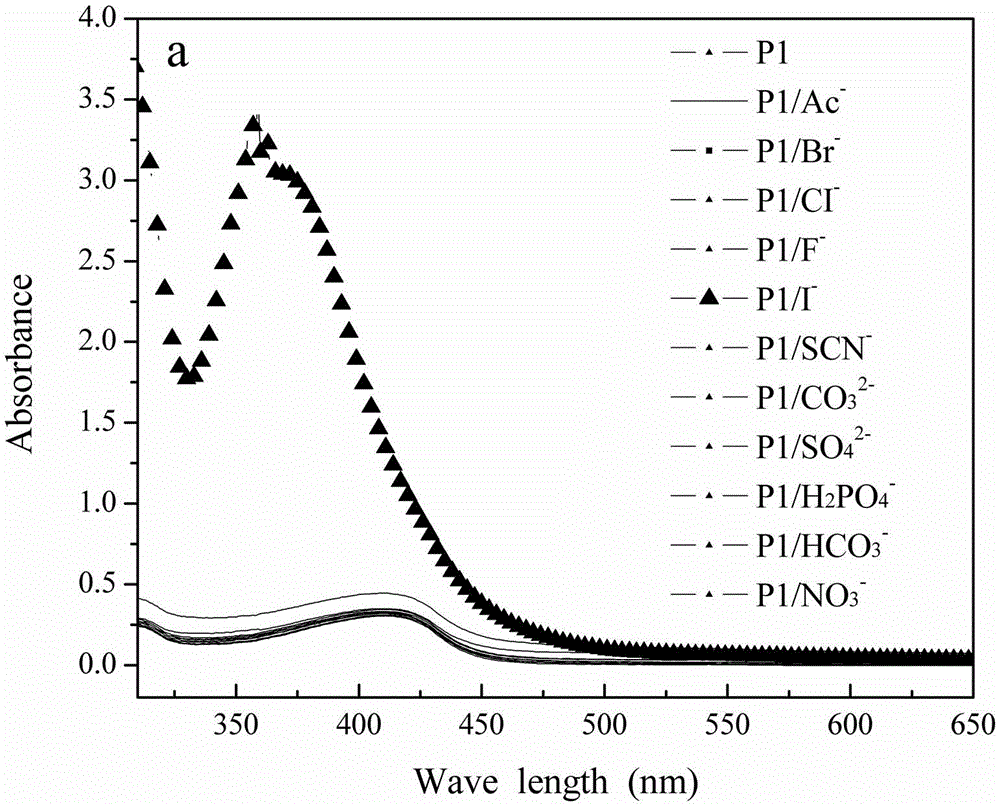
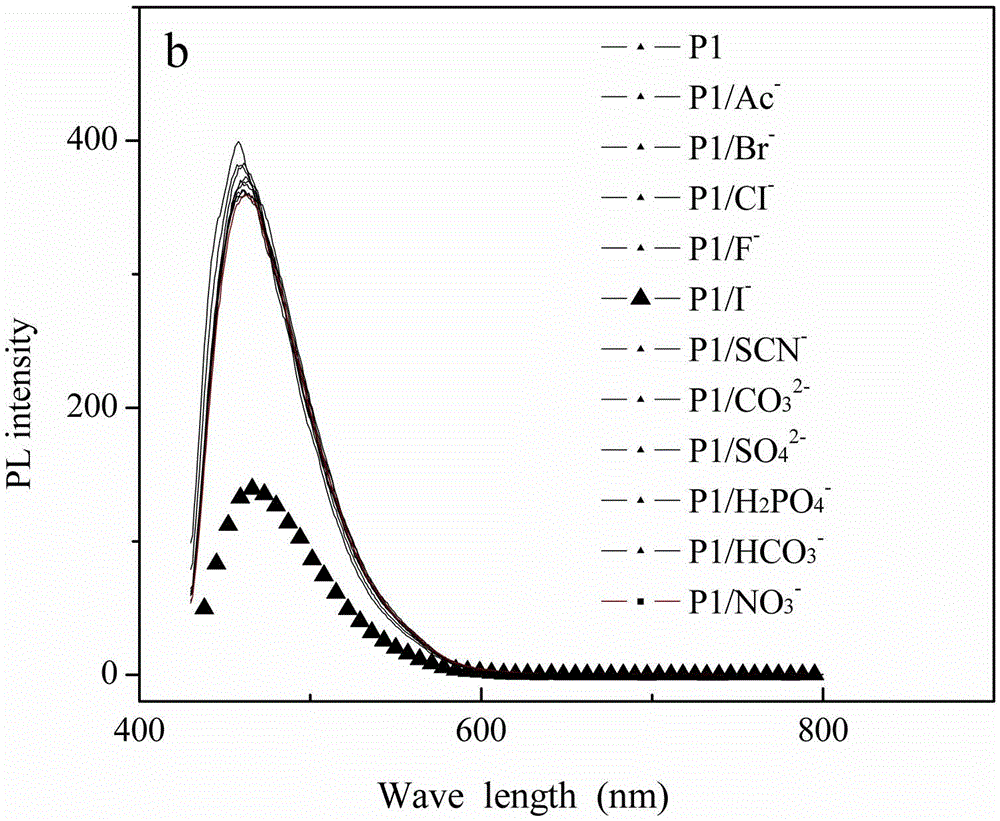
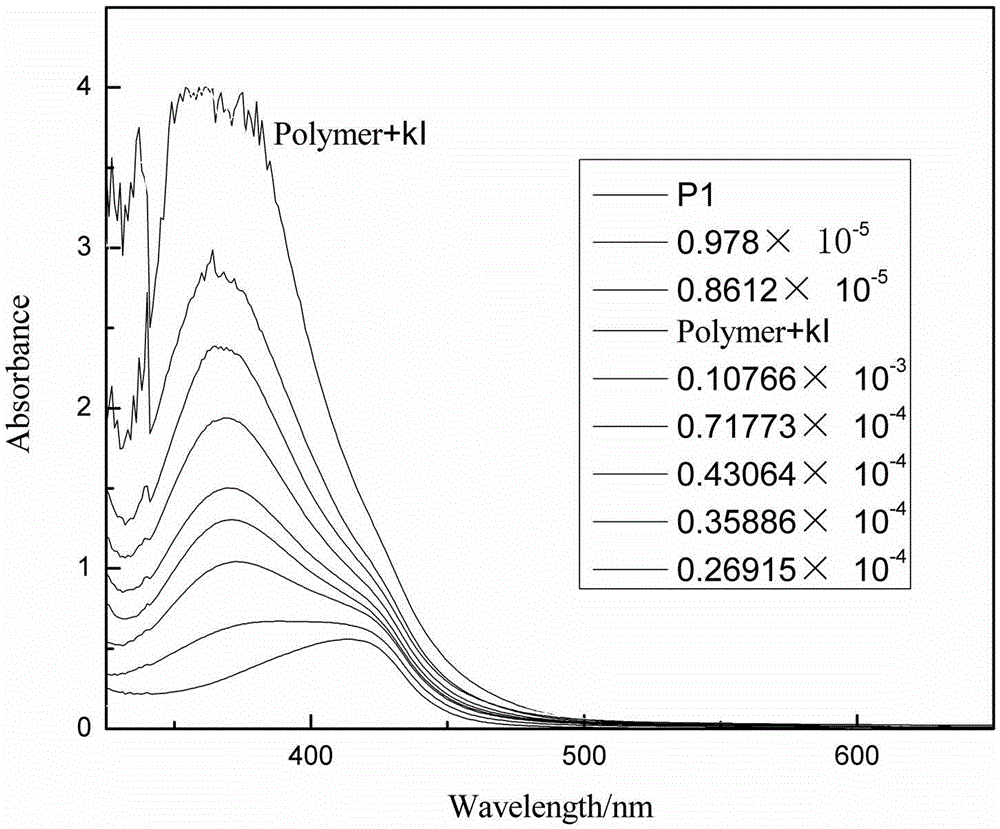
[0026] (1) Synthetic polymer: 0.165mmol1,4-diethynyl-2,5-dialkoxybenzene, 0.165mmol2-amino-4,6-dichloropyrimidine, 0.008mmolPd4(PPh3), 0.008mmolCuI under nitrogen protection At the same time, 3 ml of diisopropylamine and 7 ml of toluene were added to the reactor, and the mixture was heated at 80° C. for 24 hours to form a polymer. The polymer first emitted green light and finally turned yellowish brown. After the reaction mixture had cooled to room temperature, it was added dropwise to rapidly stirring acetone and a precipitate formed, filtered, the filter cake was collected and washed repeatedly with acetone, hot ethanol and hot methanol, and it was recrystallized twice in EtOH / CHCl3 , dried at 40°C for 48h, and the yield was 92%. The structure of the polymer was confirmed by NMR.
[0027] (2) At room temperature, I was prepared from potassium salts - , Br - , F - , CI - , Ac - and SCN - Solution, CO prepared from sodium salt 3 2- , H 2 PO 4 - , HCO 3 - , NO 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com