Benzotriazole small-organic-molecule photovoltaic material and preparation method and application thereof
A technology of benzotriazoles and photovoltaic materials, applied in photovoltaic power generation, organic chemistry, semiconductor/solid-state device manufacturing, etc., can solve the problem of low PCE of solar cell devices and lack of research on benzotriazole-based small organic molecules and other problems, to achieve the effect of improving charge transport, increasing the initial oxidation potential, and enhancing the ability to donate electrons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
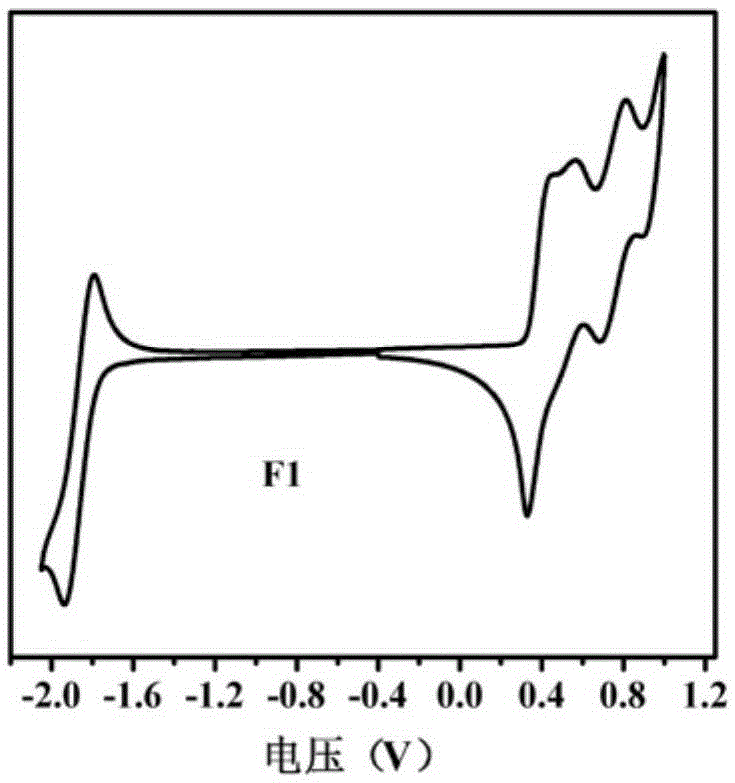
[0041] This example discloses the specific synthesis process of the organic small molecule donor material F1, including the following steps:
[0042] Under nitrogen protection, compound A (147mg, 0.2mmol), N,N-diphenyl-4-(4,4,5,5-tetramethyl-[2,1,3]Coxolane Boryl)aniline, ie D when Q represents a single bond (186mg, 0.5mmol), sodium carbonate (848mg, 8.0mmol) and tetrakis(triphenylphosphine)palladium (22mg, 0.02mmol) were dissolved in toluene (8mL) , a mixed solution of deionized water (4 mL) and ethanol (2 mL), and reflux at 110° C. for 24 hours. After the reaction solution was cooled to room temperature, it was poured into 20 mL of deionized water and separated, and extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate. The organic solvent was removed by rotary evaporation, and the crude product was purified by column chromatography using petroleum ether / dichloromethane (v:v, 5:1) as a developing solvent to obtain the target product as a...
Embodiment 2
[0049] This example discloses the specific synthesis process of the organic small molecule donor material F2, including the following steps:
[0050] Compound A (147mg, 0.2mmol), 4-(N,N-diphenylamino)styrene, that is, D (136mg, 0.5mmol) when Q represents C=C, sodium acetate (410mg, 5mmol), palladium acetate (4mg, 0.02mmol) and TBAB (26mg, 0.08mmol) were dissolved in 12mL of N,N-dimethylformamide solution and refluxed at 100°C for 36 hours. After the reaction solution was cooled to room temperature, it was poured into 50 mL of deionized water, extracted with dichloromethane, and the obtained organic phase was washed with a large amount of deionized water, and the organic phase was dried with anhydrous sodium sulfate. The organic solvent was removed by rotary evaporation, and the crude product was purified by column chromatography using petroleum ether / dichloromethane (v:v, 3:1) as a developing solvent to obtain the target product as an orange-red solid with a yield of 42%.
[...
Embodiment 3
[0057] This example discloses the specific synthesis process of the organic small molecule donor material F3, including the following steps:
[0058] Compound A (147mg, 0.2mmol), 4-(N,N-diphenylamino)phenylacetylene, that is, D (134mg, 0.5mmol) when Q represents C≡C, di(triphenylphosphine) dichloride Palladium (4mg, 0.005mmol) and cuprous iodide (2mg, 0.01mmol) were dissolved in a mixed solution of 20mL tetrahydrofuran and 15mL triethylamine, and refluxed at 70°C for 24 hours. The reaction solution cooled to room temperature was poured into 50 mL of deionized water, extracted with dichloromethane, and the combined organic phases were dried over anhydrous sodium sulfate. The organic solvent was removed by rotary evaporation, and the crude product was purified by column chromatography using petroleum ether / dichloromethane (v:v, 5:1) as a developing solvent to obtain the target product as an orange solid with a yield of 45%.
[0059] The reaction formula of above-mentioned prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fill factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com