Probe method detecting human TERT gene promoter mutation and reagent kit thereof
A promoter and human detection technology, applied in the direction of determination/inspection of microorganisms, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
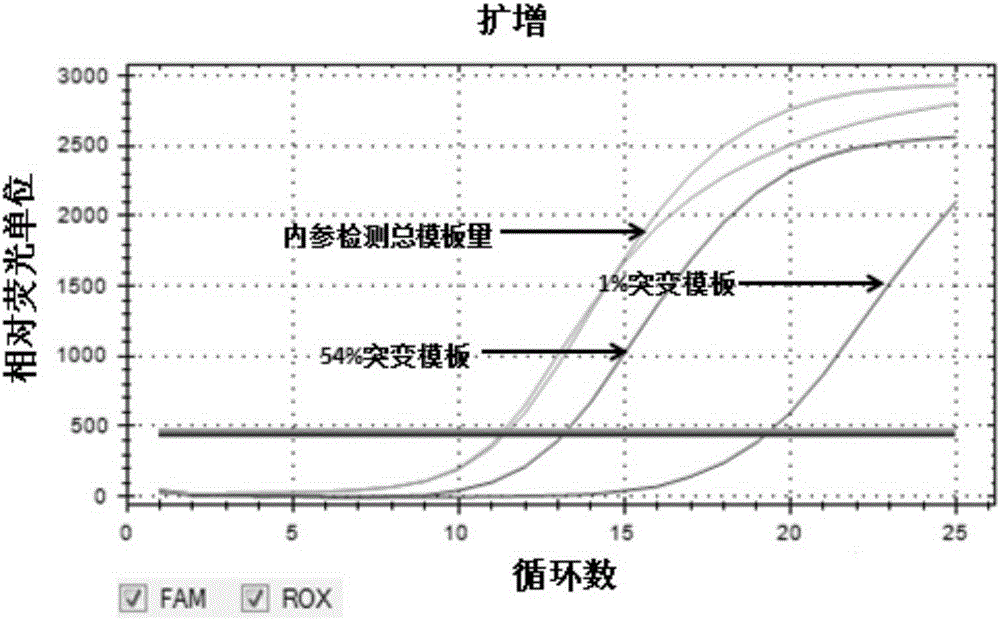
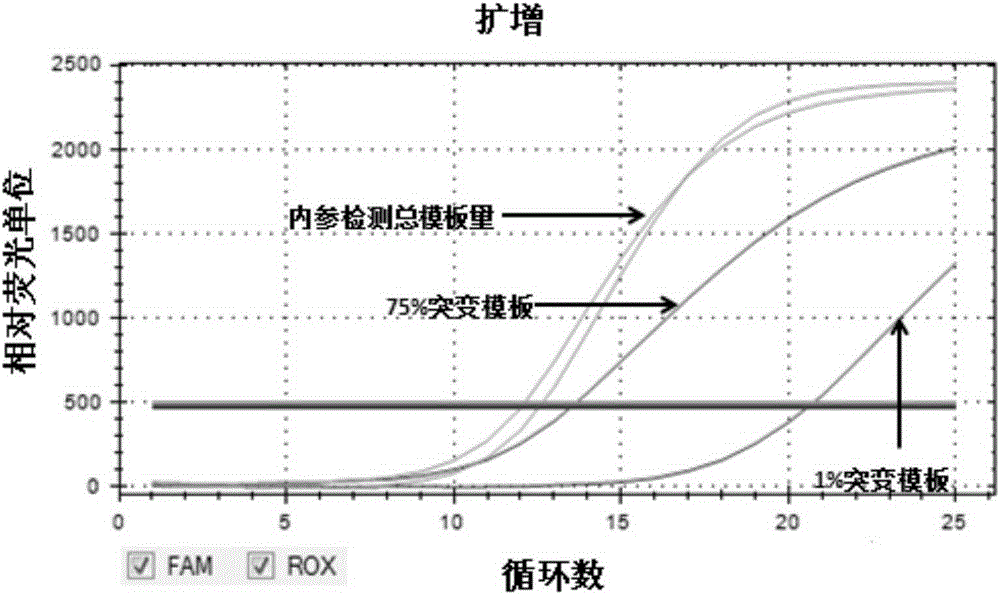
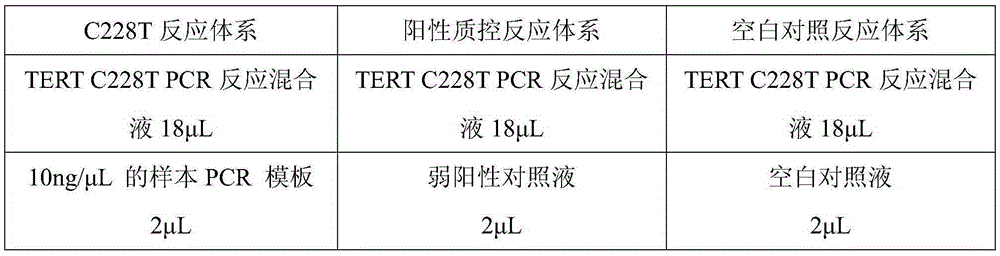
[0046] Composition of the kit
[0047] The kit of the present invention is a kit for detecting mutations of TERTC228T and TERTC250T gene promoters, including: TERTC228TPCR reaction mixture, TERTC250TPCR reaction mixture, weak positive control solution and blank control solution, wherein:
[0048] (1) TERTC228TPCR reaction mixture is to add various substances at the following concentrations into the reaction premix TakaraPremixExTaq (ProbeqPCR):
[0049] TERT C228T upstream specific primer
3μM
TERT Universal Primer
3μM
TERT Universal Probe
3μM
internal reference upstream primer
2μM
Downstream primers for internal reference
2μM
internal reference probe
2μM
dUTP
0.2μM
0.01U / μl
[0050] (2) TERTC250TPCR reaction mixture is to add various substances at the following concentrations into the reaction premix TakaraPremixExTaq (ProbeqPCR):
[0051] TERT C2...
Embodiment 2
[0088] Embodiment 2 Fluorescent PCR method contrasts the methodology verification of Sanger
[0089] Fluorescent PCR detection was carried out according to the method in Example 1, and Sanger verification was carried out at the same time. The verification data and results were as follows: 95 cases of glioma patients were randomly selected from frozen tissue samples to compare qPCR with the Sanger method in parallel, and it was obtained that qPCR and the Sanger method were consistent Rate verification results. According to the research results of clinical samples of glioma patients obtained so far, a total of 93 cases of effective detection data have been obtained. Compared with the Sanger detection methodology, the verification results show that the consistency rate between qPCR detection and Sanger verification is 100% for C228T and 100% for C250T.
[0090]The samples used for comparison were derived from glioma samples, including: anaplastic oligodendroglioma, astrocytoma,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com