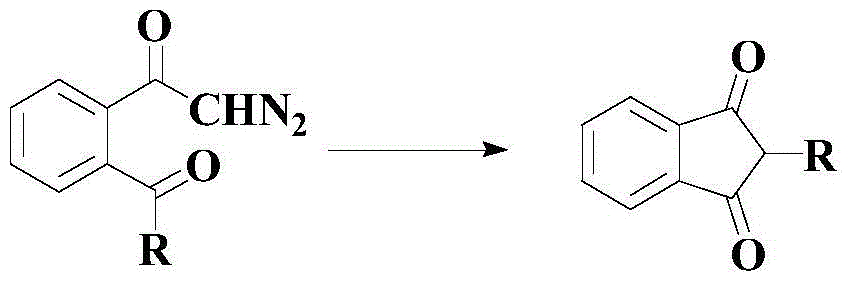A kind of catalytic synthesis method of indane-1,3-diketone compound
A synthesis method and indane technology are applied in the preparation of organic compounds, organic compounds/hydrides/coordination complex catalysts, preparation of carbon-based compounds, etc., and can solve the synthetic requirements and reaction conditions of organic synthetic pharmaceutical compounds that cannot be met. Harshness, long reaction time and other problems, to achieve the effect of satisfying industrial production, improving yield and realizing high-speed conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] At room temperature, to an appropriate amount of organic solvent DMAc (N,N-dimethylacetamide), add 100 mmol of the above formula (I) compound, 8 mmol of catalyst PdCl 2 (Py) 2 , 150mmol oxidant silver trifluoroacetate, 10mmol organic ligand L1 and 15mmol auxiliary agent triethanolamine borate, and then replace it with nitrogen several times until the reaction atmosphere is nitrogen atmosphere; raise the temperature to 80°C, and fully Stirring and reacting for 9 hours;
[0033] After the reaction, the reaction liquid was naturally cooled to room temperature, filtered, and the pH value of the filtrate was adjusted to neutral, washed with saturated brine, then extracted with ethyl acetate for 2-3 times, combined with organic phases, dried over anhydrous sodium sulfate, Concentration under reduced pressure, the resulting residue was subjected to 300-400 mesh silica gel column chromatography, and eluted with a mixture of dichloromethane and acetone at a volume r...
Embodiment 2
[0036] Reaction formula is with embodiment 1, and concrete reaction process is as follows:
[0037] At room temperature, add 100 mmol of the above formula (I) compound, 10 mmol of the catalyst PdCl to an appropriate amount of organic solvent DMAc (N,N-dimethylacetamide) 2 (Py) 2 , 200mmol oxidant silver trifluoroacetate, 14mmol organic ligand L1 and 20mmol auxiliary agent triethanolamine borate, and then replace it with nitrogen several times until the reaction atmosphere is nitrogen atmosphere; raise the temperature to 85°C, and fully Stirring and reacting for 8 hours;
[0038]After the reaction, the reaction liquid was naturally cooled to room temperature, filtered, and the pH value of the filtrate was adjusted to neutral, washed with saturated brine, then extracted with ethyl acetate for 2-3 times, combined with organic phases, dried over anhydrous sodium sulfate, Concentration under reduced pressure, the resulting residue was subjected to 300-400 mesh silica gel column c...
Embodiment 3
[0041] Reaction formula is with embodiment 1, and concrete reaction process is as follows:
[0042] At room temperature, add 100 mmol of the above formula (I) compound, 12 mmol of catalyst PdCl to an appropriate amount of organic solvent DMAc (N,N-dimethylacetamide) 2 (Py) 2 , 250mmol oxidant silver trifluoroacetate, 18mmol organic ligand L1 and 25mmol auxiliary agent triethanolamine borate, and then replace it with nitrogen several times until the reaction atmosphere is nitrogen atmosphere; raise the temperature to 90°C, and fully Stirring and reacting for 6 hours;
[0043] After the reaction, the reaction liquid was naturally cooled to room temperature, filtered, and the pH value of the filtrate was adjusted to neutral, washed with saturated brine, then extracted with ethyl acetate for 2-3 times, combined with organic phases, dried over anhydrous sodium sulfate, Concentration under reduced pressure, the resulting residue was subjected to 300-400 mesh silica gel column chro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com