Constrained-configuration dinuclear metallocene, and preparation method and application thereof
A metallocene compound and configuration technology, which is applied in the field of configuration-limited dinuclear metallocene compounds and their preparation, can solve the problems of increasing alpha-olefin steric hindrance, long steps, expensive raw materials, etc., and achieves stable steric configuration and good regulation. , the effect of good catalytic activity and copolymerization ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] The present embodiment provides a kind of restricted configuration binuclear metallocene compound, and the molecular formula of this compound (complex 1) is: [(tBuNTiCl 2 )(η 5 -C 5 h 4 )C(H)] 2 [(CH 2 ) 3 ].
[0078] The synthetic route of complex 1 is as follows:
[0079]
[0080] The specific preparation process of complex 1 is:
[0081] (1) Under the protection of argon, use methanol as a solvent, mix glutaraldehyde (100g, 1.0mol) and tetrahydropyrrolidine (3.5g, 0.05mol), cool the reaction bottle to 0°C, and slowly add new cracking Cyclopentadiene (132g, 2.0mol), after the dropwise addition, continue to stir for 4 hours. After the reaction, add a saturated saline solution of dilute acetic acid to adjust the pH to weak acidity. Remove the solvent methanol on a rotary evaporator. Extract with ether, combine the organic phases, wash with saturated NaCl, anhydrous MgSO 4 Drying; filtering, removing the residual solvent under reduced pressure to obtain a tot...
Embodiment 2
[0087] This example provides the application of the complex 1 prepared in Example 1 as a catalyst in homogeneously catalyzed ethylene polymerization.
[0088] Homogeneously catalyzed ethylene polymerization comprises the following steps:
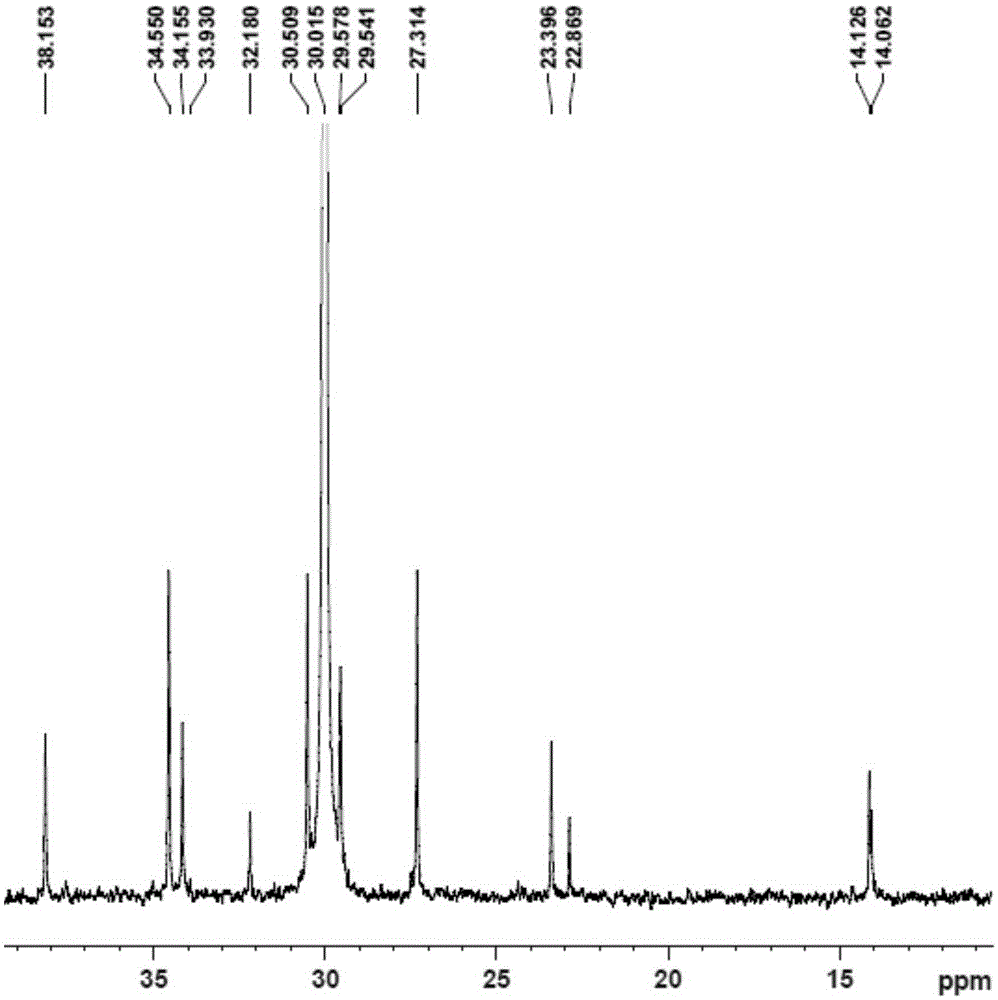
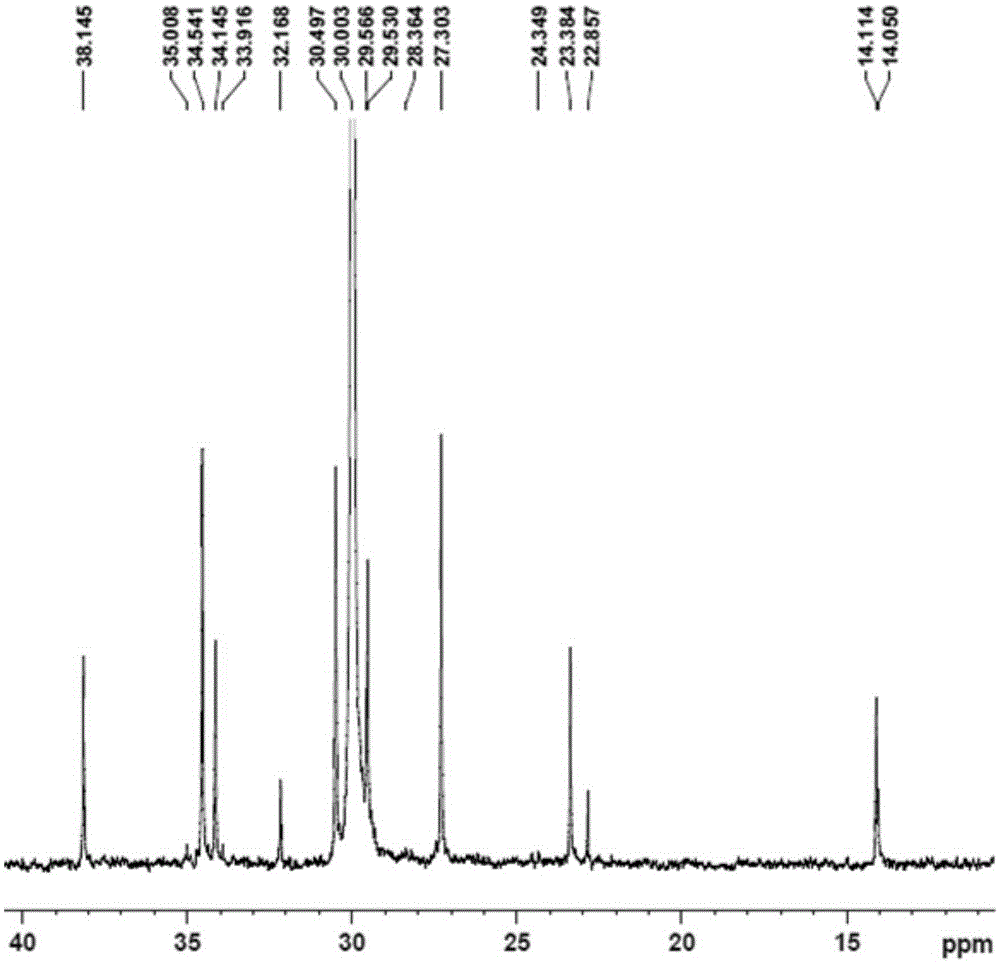
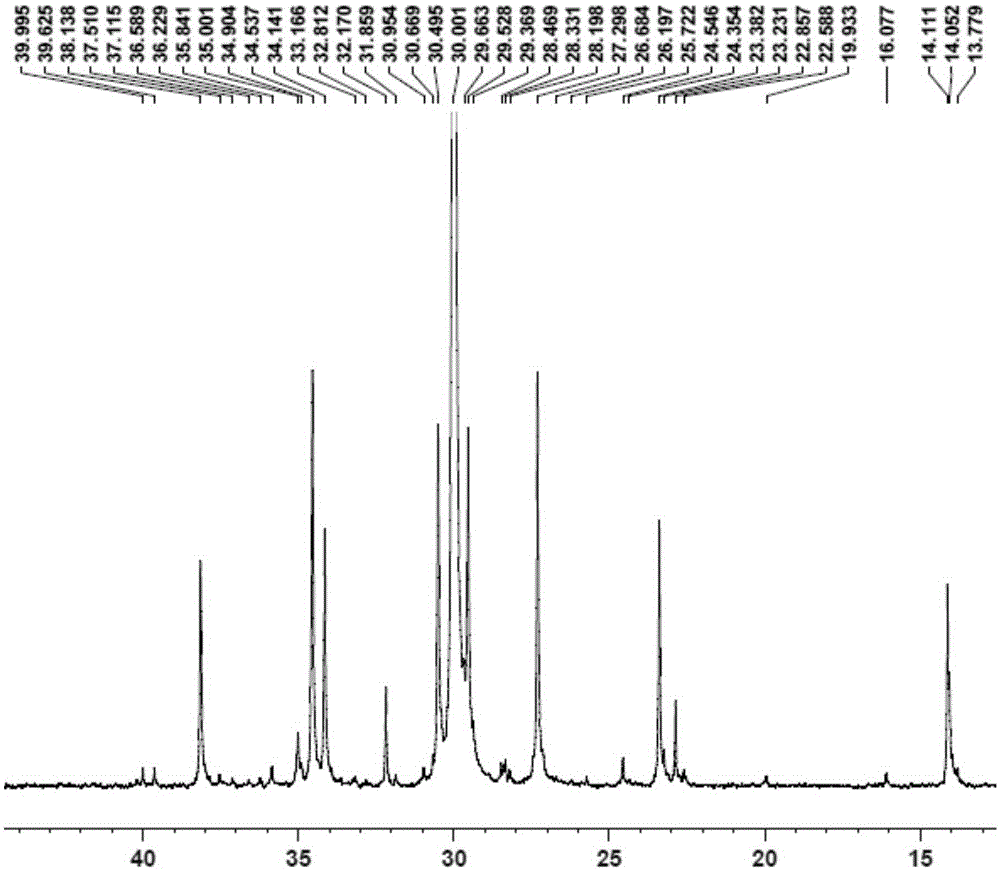
[0089] Replace the 100mL autoclave equipped with a magnetic stirrer and air duct with ethylene gas for 3 times, under the protection of nitrogen, add toluene, cocatalyst MAO2.5mL (1.60M)[Al / M=1000], 2.0μmol complexation Material 1, control the total volume to 100mL, feed ethylene gas, start the polymerization reaction at 50°C, maintain the ethylene pressure at 1.2MPa, stir the reaction for 30min, close the gas cylinder, release the pressure, and then terminate the reaction with 10% hydrochloric acid ethanol. Transfer the polymer to a beaker, let it stand overnight, filter and wash the polymer fully with ethanol, dry it in vacuum at 60°C to constant weight, weigh the polymer mass, and calculate that the polymerization activity of the catalyst...
Embodiment 3
[0091] This example provides the application of the complex 1 prepared in Example 1 as a catalyst in the homogeneously catalyzed copolymerization of ethylene and 1-hexene.
[0092] Homogeneously catalyzed ethylene and 1-hexene copolymerization comprises the following steps:
[0093] Replace the 100mL autoclave equipped with a magnetic stirrer and gas tube with ethylene gas for 3 times, and add toluene, 1-hexene 10mL, cocatalyst MAO2.5mL (1.60M) [Al / M=1000 ], 2.0μmol complex 1, control the total volume to 100mL, feed ethylene gas, start the polymerization reaction at 50°C, maintain the ethylene pressure at 0.3MPa, stir the reaction for 30min, close the cylinder, and terminate the reaction with 10% hydrochloric acid ethanol . Transfer the polymer to a beaker, let it stand overnight, filter and wash the polymer fully with ethanol, dry it in vacuum at 60°C to constant weight, weigh the polymer, and calculate the catalyst polymerization activity to be 1.1×10 6 gpolymer / molM h, Mw...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com