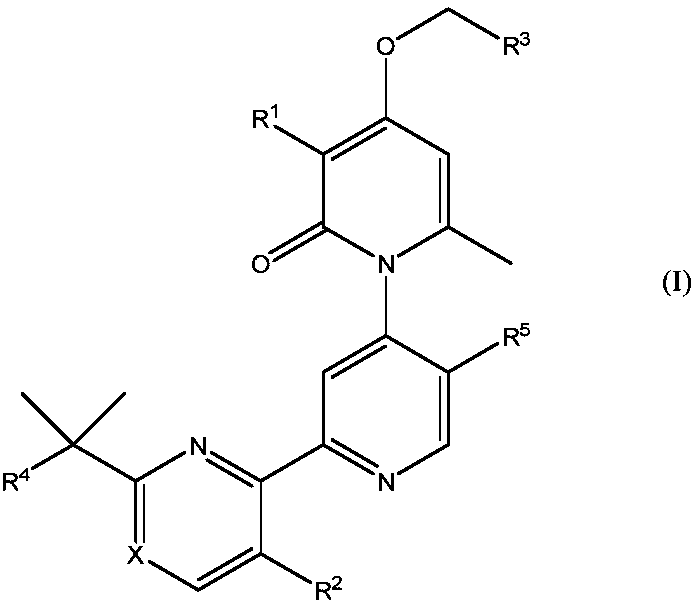
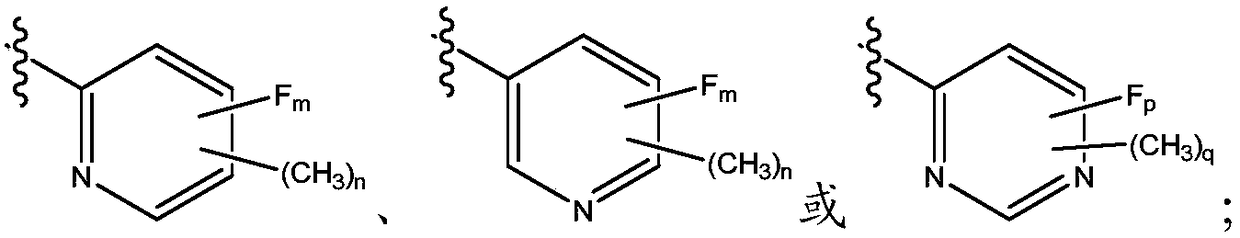
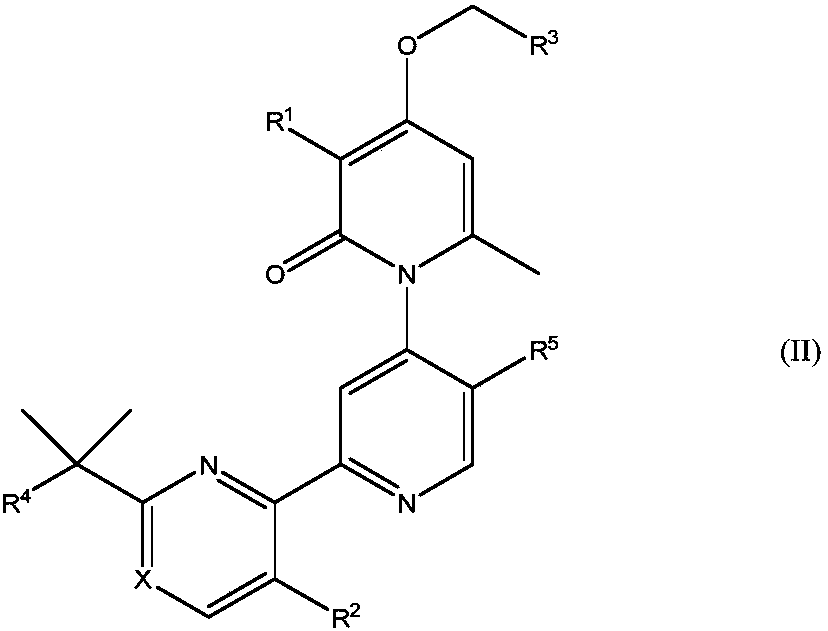
Methyl/fluoro-pyridyl-methoxy substituted pyridone-pyridyl compounds and fluoro-pyrimidinyl-methoxy substituted pyridone-pyridyl compounds
A picoline and methyl pyrimidine technology, applied in the treatment of p38 kinase-mediated diseases, disease treatment, pyridone-pyridyl compounds, can solve the problems of difficult dose adjustment, neutralizing antibody response, high treatment costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment B
[0273] Compound No. 65, Example B: 3-Chloro-4-((5-fluoropyrimidin-4-yl)methoxy)-2'-(2-(2-hydroxypropan-2-yl)pyrimidine-4- base)-5',6-dimethyl-2H-[1,4'-bipyridyl]-2-one
[0274]
[0275] The title compound was prepared according to Compound 49, Example A through Step E, but substituting 4-(chloromethyl)-5-fluoropyrimidine for 2-(chloromethyl)-3,5-difluoropyridine to make 3-chloro -4-Hydroxy-2'-(2-(2-hydroxyprop-2-yl)pyrimidin-4-yl)-5',6-dimethyl-2H-[1,4'-bipyridine]-2 - Alkylation of ketones to produce the desired dibenzyl ethers. The title compound was prepared according to the general procedure of Compound 49, Step F of Example A.
[0276] E. Treatment
[0277] The present disclosure also provides methods for treating a condition in a patient suffering from or susceptible to such a condition by administering to the subject a therapeutically effective amount of one or more compounds as described above. In one embodiment, the treatment is prophylactic treatment. In anot...
Embodiment C
[0341]Example C: p38 Inhibition Potency and p38 / MK2 Substrate Selectivity: This study evaluated the potency of compounds of the invention in inhibiting the p38 pathway. p38 activates MK2 and PRAK through phosphorylation, which then interact with Hsp27, leading to increased inflammation and reduced ability to handle shock. This study examined half the amount of compounds of the invention required to inhibit MK2 and PARK activation. This is a measure of the effectiveness of the compounds of the invention in helping to reduce the inflammatory response, which is useful in the treatment of many diseases, including autoimmune conditions, lymphoma and rheumatoid arthritis. The novel MK2 substrate selective inhibition mechanism of compounds was evaluated in an enzymatic assay comparing the potency of inhibitors in blocking p38 / MK2 versus p38 / PRAK induced phosphorylation of HSP-27 derived peptide substrates. The ability of compounds to inhibit activated phosphorylated p38α was evaluat...
Embodiment D
[0344] Example D: Cytokine regulation in human monocytes: The p38 pathway has been shown to be critical for the biosynthesis of several pro-inflammatory cytokines including TNFα, IL-1β and IL-6. Thus, inhibition of the p38 MAPK pathway reduces inflammatory responses by reducing the biosynthesis of pro-inflammatory cytokines. This study shows half the amount of the compounds of the invention required to inhibit the biosynthesis of TNF[alpha], IL-6 and IL-1[beta] (pro-inflammatory cytokines). This is a reflection of the effect of the compounds of the invention in helping to reduce inflammation, which is useful in the treatment of many diseases, including autoimmune conditions, lymphomas and rheumatoid arthritis. The potency and efficacy of p38 inhibitors to block cytokine production was evaluated using the human U937 cell line. The U937 human promonocytic cell line was obtained from the American Type Culture Collection (Rockville, MD). These cells differentiate into monocyte p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com