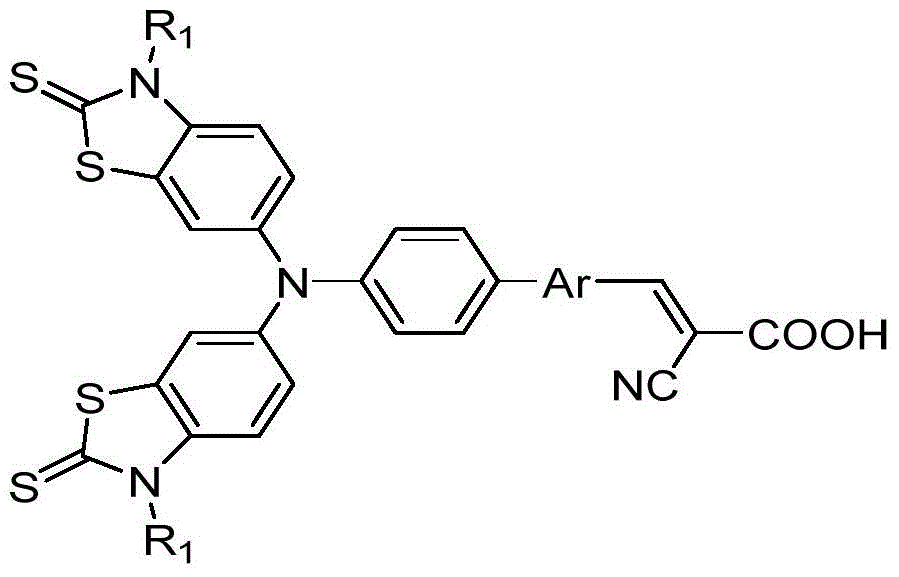
A kind of thiazolethione triphenylamine photosensitive dye and preparation method thereof
A technology of photosensitive dyes and thiazolethiones, which is applied in the field of organic dyes, can solve the problems of destroying the coplanarity of molecules, narrowing the spectral absorption range of photosensitizing dye molecules, and unfavorable electron transfer, so as to achieve low preparation costs, improve electron donating ability, The effect of broadening the spectral absorption range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Take the preparation of thiazolethioketotriphenylamine photosensitizing dyes with the following structural formula as an example, the preparation method is as follows:
[0036]
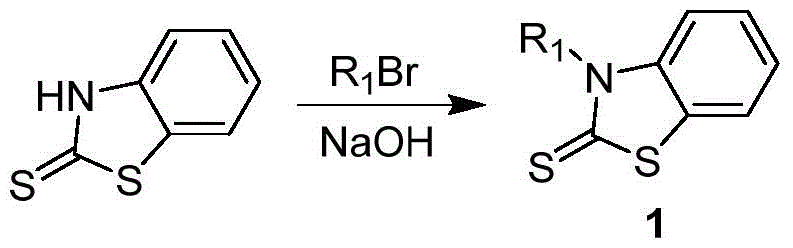
[0037] 1, preparation formula 1 compound
[0038] Add 300mL toluene and 300mL distilled water to a three-neck round bottom flask, then add 50.1g (0.3mol) benzothiazolethione, 54.0g (0.33mol) bromohexane, 13.2g (0.33mol) sodium hydroxide, 9.6g ( 0.03mol) tetrabutylammonium bromide, react at 60°C for 12 hours, cool down to room temperature, separate liquids, wash the organic phase with distilled water until neutral, dry with anhydrous magnesium sulfate, filter, evaporate the solvent, and use the column layer Analysis and separation (eluent is the mixed solution of sherwood oil:ethyl acetate=2:1), obtains formula 1 compound 66.3g, and its yield is 88%, and chemical reaction equation is as follows:
[0039]
[0040] 2. Preparation of the compound of formula 2
[0041] Add 400mL of chlorofor...
Embodiment 2
[0061] Take the preparation of thiazolethioketotriphenylamine photosensitizing dyes with the following structural formula as an example, the preparation method is as follows:
[0062]
[0063] In the step 1 of the preparation of the compound of formula 1 in Example 1, the hexyl bromide used is replaced with equimolar methyl iodide, the other steps of this step are the same as in Example 1, and the other steps are the same as in Example 1 to prepare thiazolethione The triphenylamine photosensitive dye has an ultraviolet absorption range of 300-612nm and a maximum absorption wavelength of 450nm.
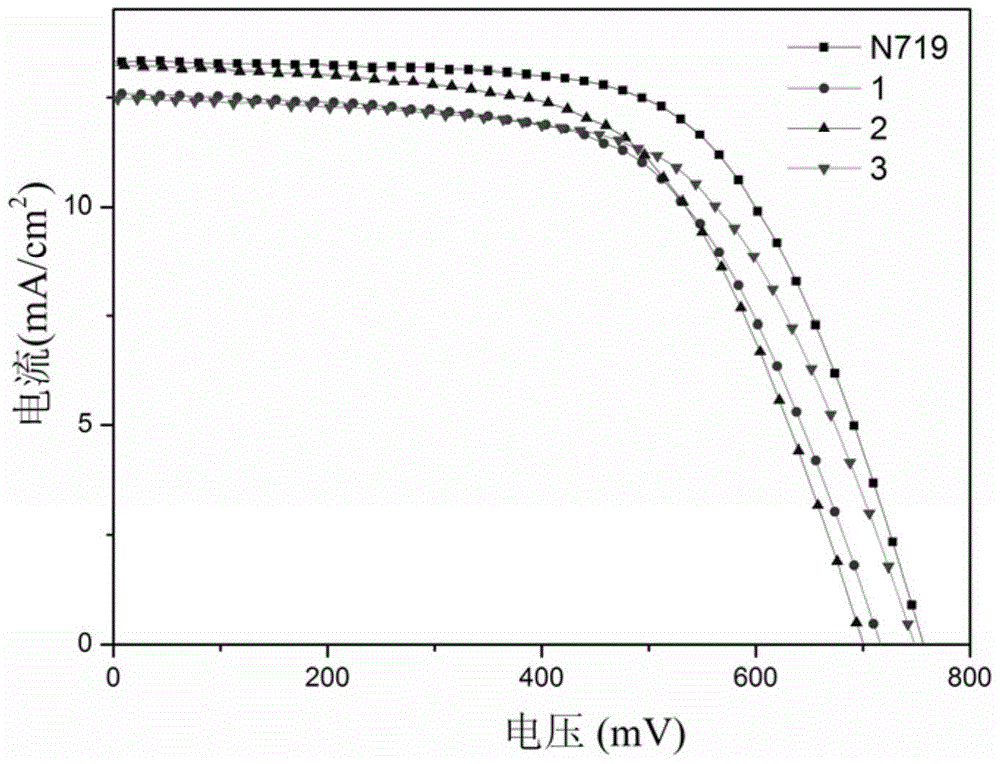
[0064] According to the method of Example 1, the thiazolethioketotriphenylamine photosensitive dye of this example was prepared into a dye-sensitized solar cell, and the photoelectric conversion efficiency was 6.14% and the short-circuit current was 13.1mA / cm after testing. 2 , the open circuit voltage is 700mV, and the fill factor is 0.67 (see figure 1 Middle curve 2), its photoel...
Embodiment 3
[0066] Take the preparation of thiazolethioketotriphenylamine photosensitizing dyes with the following structural formula as an example, the preparation method is as follows:
[0067]
[0068] In step 1 of the preparation of the compound of formula 1 in Example 1, the hexyl bromide used is replaced by equimolar decane bromide, the other steps of this step are the same as in Example 1, and the other steps are the same as in Example 1 to prepare thiazole sulfur Ketotriphenylamine photosensitive dye has an ultraviolet absorption range of 300-610nm and a maximum absorption wavelength of 445nm.
[0069] According to the method of Example 1, the thiazolethioketotriphenylamine photosensitizing dye of this example was prepared into a dye-sensitized solar cell. After testing, the photoelectric conversion efficiency was 6.14%, and the short-circuit current was 12.5mA / cm 2 , the open circuit voltage is 745mV, and the fill factor is 0.66 (see figure 1 Middle curve 3), its photoelectri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com