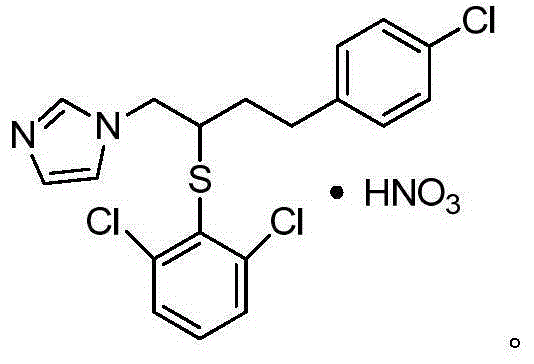
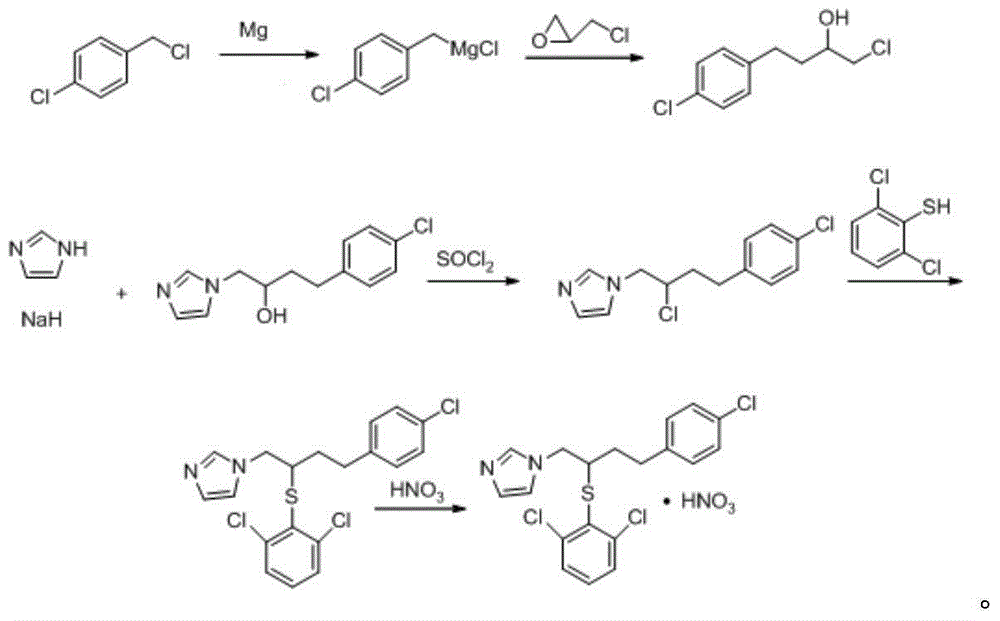
Method for industrial production of butoconazole nitrate
A technology for butconazole nitrate and imidazole, applied in the field of chemical synthesis technology, can solve the problems of lack of impurities, effective control, complicated post-processing steps, etc., and achieve the effects of easy purification and purification, reducing the generation of impurities, and improving the purity and yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Follow the steps below to produce butoconazole nitrate:
[0068] (1) Take 6kg1-(2-chloro-4-(4-chlorophenyl)butyl)-1hydro-imidazole, 6kg2,6-dichlorothiophenol, 4kg anhydrous potassium carbonate and 50kg acetone, 65℃ Under reflux reaction for 5.5 hours, add 1kg of anhydrous potassium carbonate, reflux reaction at 65°C for 7.5 hours, slowly cool with circulating water at 0-4°C, centrifuge at 2825r / min for 30min, discard the solid, keep the liquid, It is the filtrate; the filtrate is concentrated at 65°C until no acetone is evaporated to obtain a concentrate;
[0069] (2) Take the concentrate obtained in step (1), add an extract that is 10 times the weight of the concentrate, and the extract is composed of ether, acetone and water in a weight ratio of 3:3:2; stir at 20°C After 15 minutes, let it stand still, discard the aqueous phase, and set aside the organic phase;
[0070] (3) under the stirring condition of ice bath, 1.5 rev / s, in step (2) gained organic phase, add th...
Embodiment 2
[0074] Follow the steps below to produce butoconazole nitrate:
[0075] (1), (2) are with embodiment 1;
[0076] (3) under the stirring condition of ice bath, 1 rev / s, in step (2) gained organic phase, add the nitric acid that concentration is 69% with the speed of 1ml / s dropwise, to stop generating after precipitation at 2825r / min centrifuge for 20min under the condition of 2825r / min, discard the liquid and keep the solid; The retained solid was mixed with 6 times the weight of acetone, stirred thoroughly, centrifuged at a speed of 2825r / min for 20min, discarded the liquid, retained the solid, and dried at 45°C and -0.5Mpa for 3 hours to obtain the crude product of butoconazole nitrate ;
[0077] (4) with embodiment 1.
[0078] After testing, the yield of the product of this example was 87.33%. After testing, the content of the target product 1-(4-(4-chlorophenyl)-2-((2,6-dichlorophenyl)thio)butyl)-1 hydrogen-imidazole mononitrate is 99.59% , the content of impurity 1-(4...
Embodiment 3
[0080] Follow the steps below to produce butoconazole nitrate:
[0081] (1), (2) are with embodiment 1;
[0082] (3) under the stirring condition of ice bath, 2 turns / seconds, in step (2) gained organic phase, add the nitric acid that concentration is 69% with the speed of 3ml / s, to stop generating precipitation at 2825r / min after rotating speed Centrifuge for 40min under the condition of 2825r / min, discard the liquid and keep the solid; the filter cake is mixed with 4 times the weight of diethyl ether and then fully stirred. The retained solid was mixed with 8 times the weight of acetone, then fully stirred, centrifuged at a speed of 2825r / min for 40min, the liquid was discarded, the solid was retained, and dried at 55°C and 0.5Mpa for 7 hours to obtain the crude product of butoconazole nitrate;
[0083] (4) with embodiment 1.
[0084] After testing, the yield of the product in this example was 85.11%. After testing, the content of the target product 1-(4-(4-chlorophenyl)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com