1-alkylated daphnane diterpene and application thereof to preparation of anti-HIV drugs
A technology for alkylating daphne and daphne-type diterpenes, which can be used in antiviral agents, organic chemistry, pharmaceutical formulations, etc., and can solve the problem that malignant viruses cannot be completely eliminated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
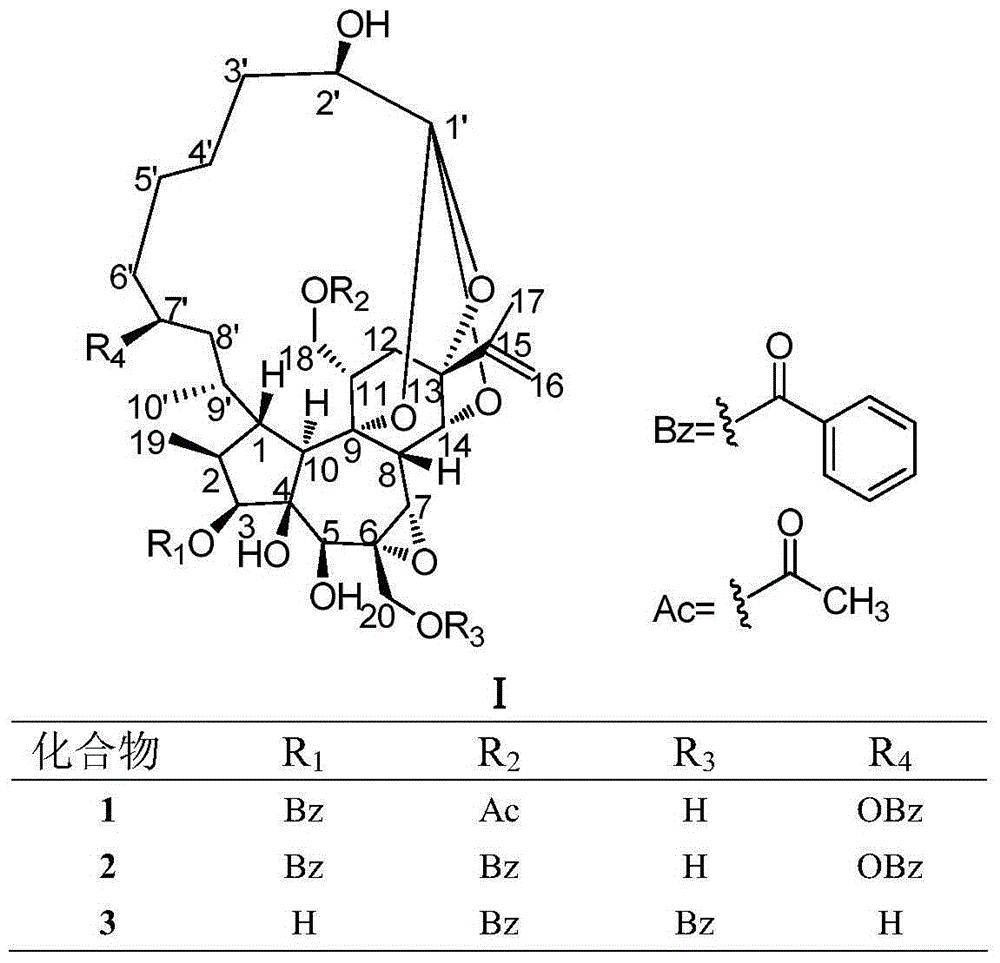
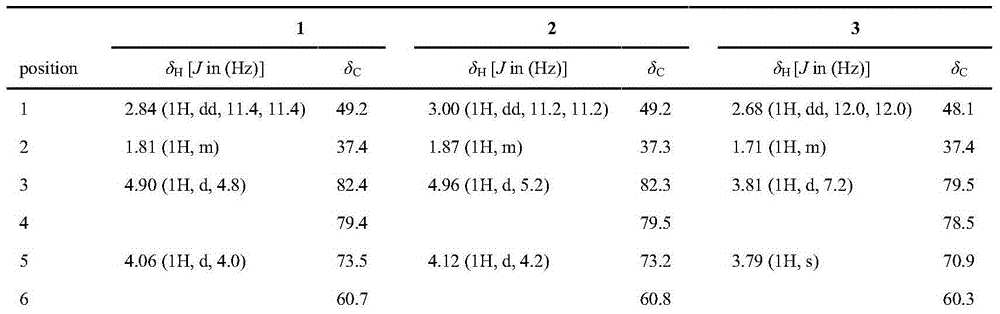
[0023] Example 1 Preparation of 1-alkylated daphne-type diterpenes in Daphne chamaejasme
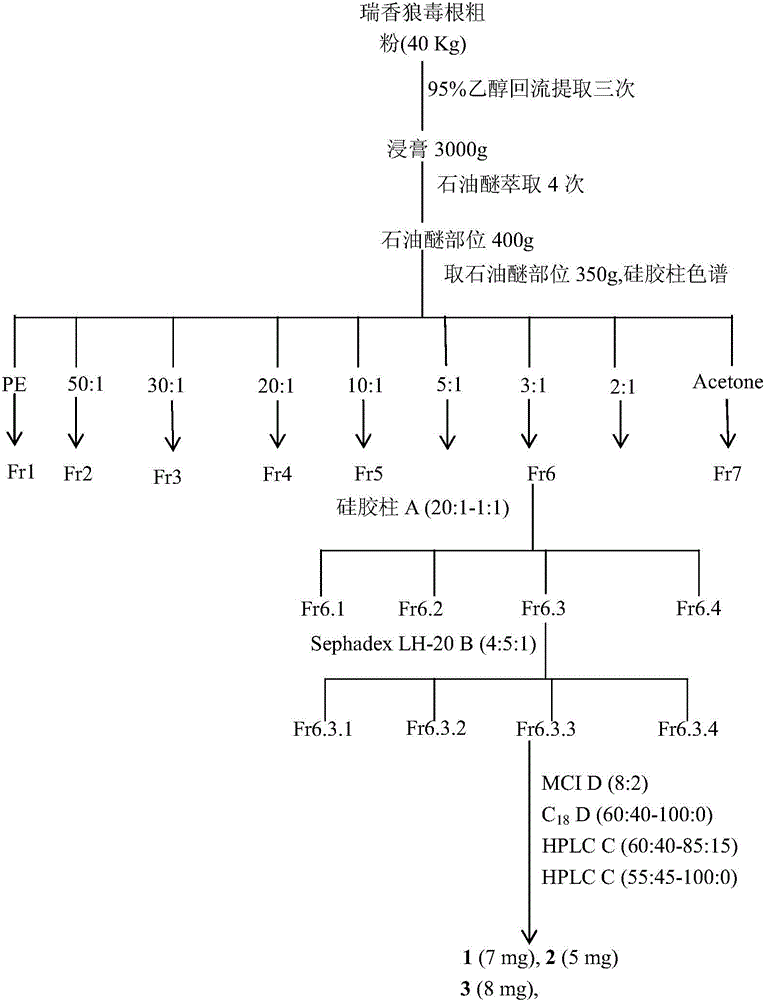
[0024] Take 40kg of Rhizoma chamaejasma root, heat and reflux with 95% ethanol to extract, concentrate the extract and extract with petroleum ether to obtain a total of 400g of extract, take 350g dry sample and carry out silica gel column chromatography, elute with petroleum ether-acetone gradient, and Petroleum ether: Acetone (5:1~2:1) Fractions recover the solvent under reduced pressure and perform rapid medium-pressure silica gel column chromatography, use petroleum ether-ethyl acetate system (20:1~10:1~5:1~2 :1~1:1) for gradient elution, a total of 15 parts of eluent were collected, each part of 2000ml, the 8th to 13th parts were combined, the solvent was recovered under reduced pressure and then separated by SephadexLH-20 column chromatography, and cyclohexane / dichloromethane / methanol (4:5:1) for elution, collected 20 parts of the eluent in total, each part of 80ml, combined the 9t...
Embodiment 2
[0025] Embodiment 2 in vitro anti-HIV experiment
[0026] Infection of MT with HIV-1 NL4-3 Strain 4 Cells (infection multiple = 0.001) were added with different concentrations of drugs at the same time. After 48 hours of infection, fresh substrates containing appropriate concentrations of drugs were added to maintain the normal growth of the cells. After 4 days, the P24ELISA kit was used to analyze the replication of the virus; the experiment was performed with Zidov Set (AZT) as the positive control drug; the EC of the compound 50 Refers to the drug concentration when the drug inhibits the production of HIV-1 P24 antigen to 50%, and the EC is obtained by linear regression analysis 50 (Biosoftsoftware), the results are shown in Table 2. Table 2 Compound 1-3 anti-HIV activity assay results
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com