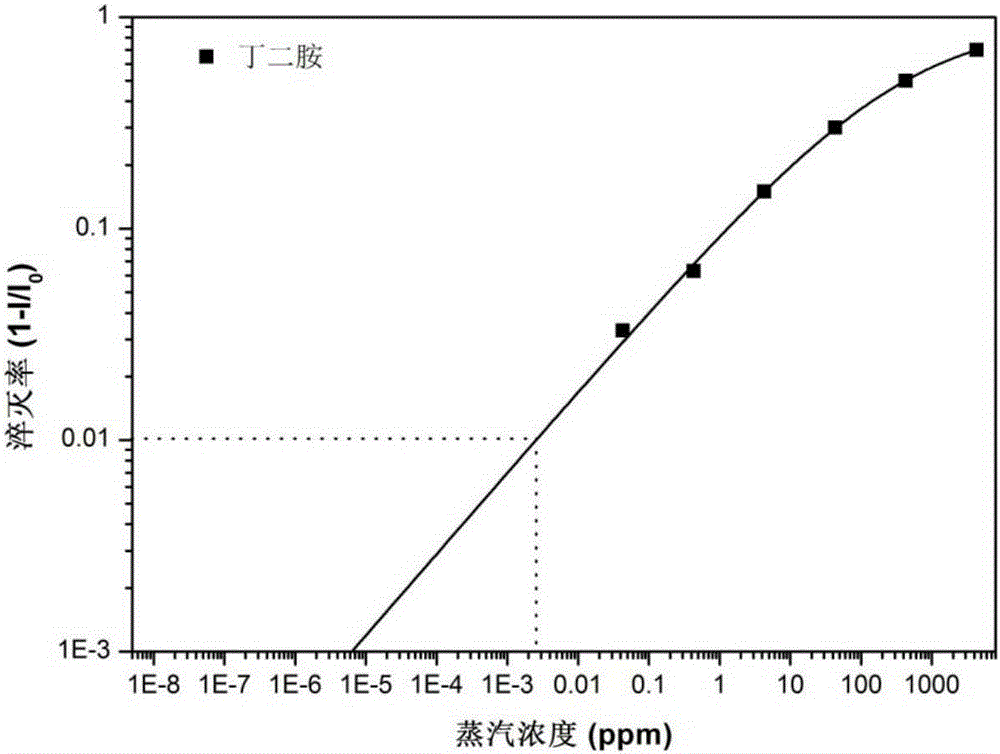
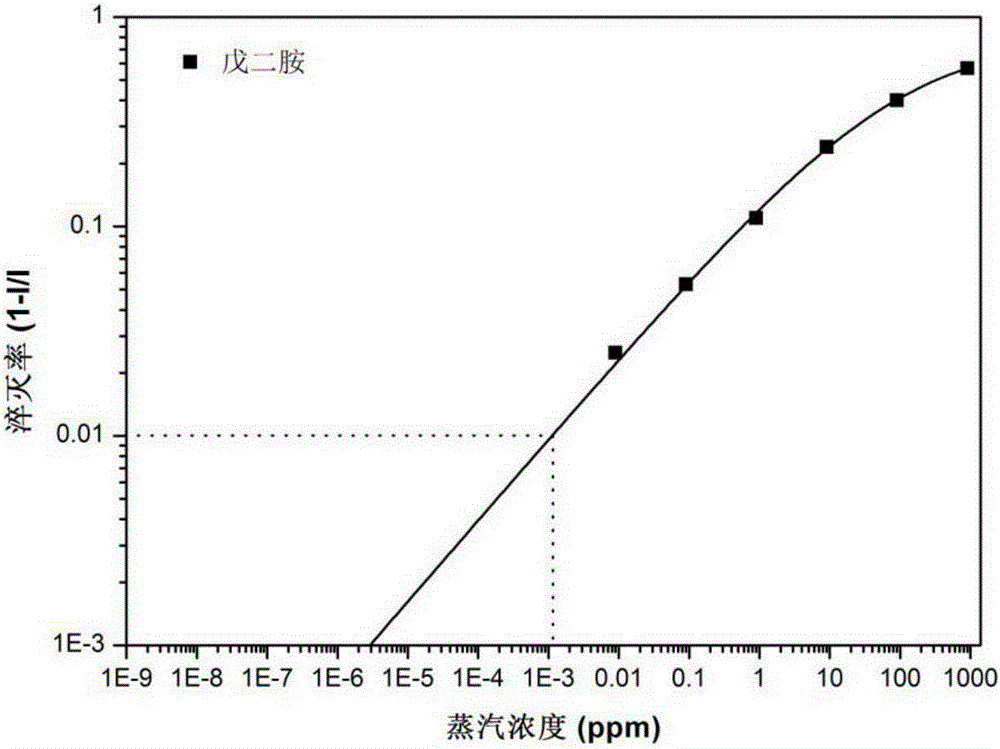
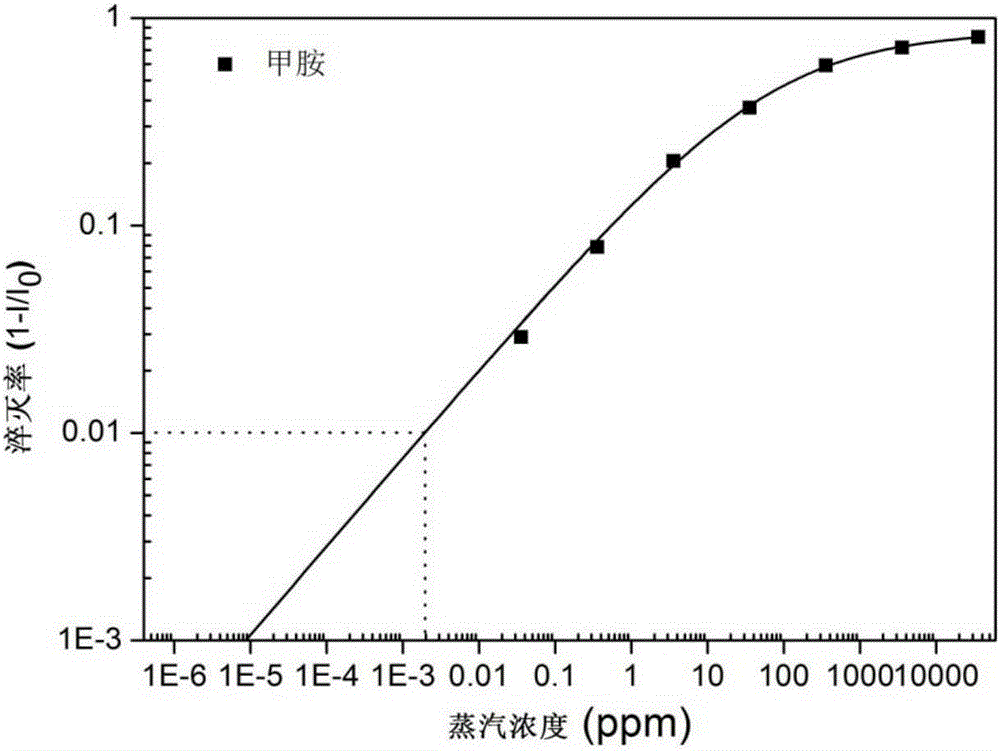
One-dimensional organic semiconductor nanotube with fluorescent response to organic amine gas and preparation method and application of one-dimensional organic semiconductor nanotube
A technology of organic semiconductors and nanotubes, applied in the application field of one-dimensional organic semiconductor nanotubes, can solve the problems of lack of porous structure, expensive preparation and processing, low quantum efficiency, etc., achieve large specific surface area, high application value, improve detection out of the limit effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] (1) Preparation of a peryleneimide derivative monomer having the following molecular formula with a 3-pentyloxyphenethyl group at one end and a dodecyl chain at the other end.
[0062]
[0063] 1.1) 100 mg of perylene-3,4,9,10-tetracarboxylic dianhydride and 10 g of imidazole were mixed and heated to 130°C to dissolve, then injected into 40 mg of dodecylamine for reaction to obtain a reaction solution, Then add 15 milliliters of ethanol and 15 milliliters of concentrated hydrochloric acid to the reaction solution and stir overnight; take out the product, wash it with water until the pH is neutral, and dry it;
[0064] 1.2) Get 100 mg of the product obtained after drying in step 1.1), add 10 grams of imidazole and 300 microliters of 3-isoamyloxyphenethylamine to it, and react at a temperature of 140° C., and then add to the obtained Add 8-15 ml of concentrated hydrochloric acid to the reaction solution, stir overnight, and take out the product to obtain a peryleneimid...
Embodiment 2
[0073] (1) Preparation of peryleneimide derivative monomers having the following molecular formula, one end of which is respectively 3-propoxybenzyl group, and the other end is substituted with a dodecyl chain.
[0074]
[0075] 1.1) 100 mg of perylene-3,4,9,10-tetracarboxylic dianhydride and 10 g of imidazole were mixed and heated to 130°C to dissolve, then injected into 40 mg of dodecylamine for reaction to obtain a reaction solution, Then add 15 milliliters of ethanol and 15 milliliters of concentrated hydrochloric acid to the reaction solution and stir overnight; take out the product, wash it with water until the pH is neutral, and dry it;
[0076] 1.2) Get 100 mg of the product obtained after drying in step 1.1), add 10 grams of imidazole and 300 microliters of 3-isopropoxybenzylamine to it, and react at a temperature of 140 ° C, and then add to the obtained Add 8-15 ml of concentrated hydrochloric acid to the reaction solution, stir overnight, and take out the product...
Embodiment 3
[0081] (1) Prepare a peryleneimide derivative monomer having the following molecular formula with a 3-pentyloxybenzyl group at one end and a dodecyl chain at the other end.
[0082]
[0083] 1.1) 100 mg of perylene-3,4,9,10-tetracarboxylic dianhydride and 10 g of imidazole were mixed and heated to 130°C to dissolve, then injected into 40 mg of dodecylamine for reaction to obtain a reaction solution, Then add 15 milliliters of ethanol and 15 milliliters of concentrated hydrochloric acid to the reaction solution and stir overnight; take out the product, wash it with water until the pH is neutral, and dry it;
[0084] 1.2) Get 100 mg of the product obtained after drying in step 1.1), add 10 grams of imidazole and 300 microliters of 3-isoamyloxybenzylamine to it, and react at a temperature of 140° C. Add 8-15 ml of concentrated hydrochloric acid to the reaction solution, stir overnight, and take out the product to obtain a peryleneimide derivative. NMR data 1 HNMR (400MHz, CD...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Wall thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com