A preparation method of minodronic acid for treating osteoporosis
A technology for minodronic acid and osteoporosis, applied in the field of medicine and chemical industry, can solve the problems of slow reaction speed, harsh conditions, and many by-products, and achieve the effects of increased reaction speed, increased yield, and easy removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
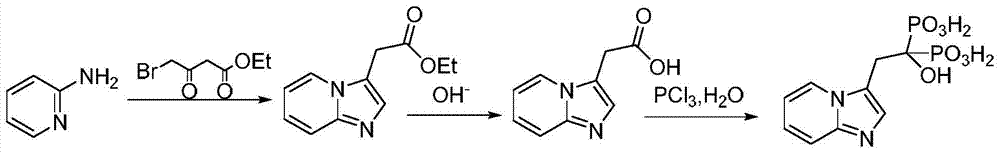
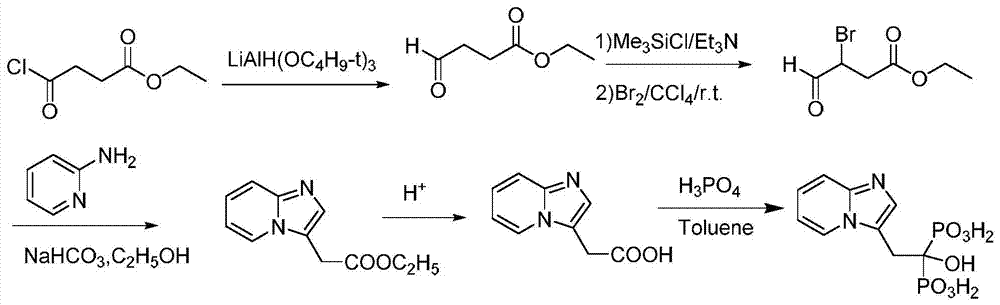
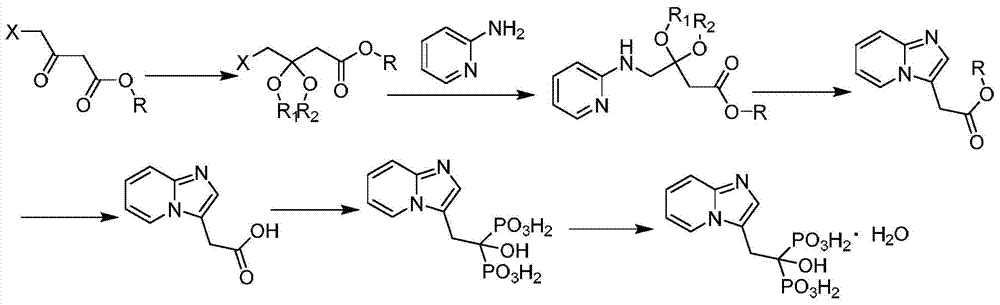
[0039] A preparation method of minodronic acid for treating osteoporosis, the method comprises the following steps:
[0040](1) 14.19g (51.8mmol) of ethyl 3,4-dibromo-butyrate and 1.42g of reaction accelerator A were added into a three-necked flask of a mixed solvent of water and 1,4-dioxane in 100ml, Stir at room temperature for 15 minutes, then add 5.85g (61.16mmol) of 2-aminopyridine and stir at 80°C for 3 hours. TLC monitors that the reaction of ethyl 3,4-dibromo-butyrate is complete, filter, concentrate under reduced pressure, and add water , extracted with ethyl acetate (50mL×3 times), combined the organic phases, removed the solvent under reduced pressure, and dried to give 10g of light yellow oil, which was 2-(imidazo[1,2-α]pyridin-3-yl)acetic acid Ethyl ester, yield 93.1%, purity 98.5%; Among them, the reaction accelerator A is composed of zinc nitrate and glycine with a weight ratio of 4:1, and the mixed solvent is formed by mixing 10ml water and 30ml 1,4-dioxane . ...
Embodiment 2
[0044] A preparation method of minodronic acid for treating osteoporosis, the method comprises the following steps:
[0045] (1) 14.19g (51.8mmol) of ethyl 3,4-dibromo-butyrate and 1.14g of reaction accelerator A were added in a three-necked flask of a mixed solvent of water and 1,4-dioxane in 100ml, Stir at room temperature for 15 minutes, then add 6.34g (67.34mmol) of 2-aminopyridine and stir at 85°C for 5 hours. TLC monitors that the reaction of ethyl 3,4-dibromo-butyrate is complete, filter, concentrate under reduced pressure, and add water , extracted with ethyl acetate (50mL×3 times), combined the organic phases, removed the solvent under reduced pressure, and dried to give 10.2g of a light yellow oil, which was 2-(imidazo[1,2-α]pyridin-3-yl) Ethyl acetate, the yield is 94.7%, and the purity is 98.2%. Among them, the reaction accelerator A is composed of zinc nitrate and glycine with a weight ratio of 4.5:1, and the mixed solvent is formed by mixing 15ml of water and 30m...
Embodiment 3
[0049] A preparation method of minodronic acid for treating osteoporosis, the method comprises the following steps:
[0050] (1) 14.19g (51.8mmol) of ethyl 3,4-dibromo-butyrate and 1.28g of reaction accelerator A were added in a three-necked flask of a mixed solvent of water and 1,4-dioxane in 100ml, Stir at room temperature for 15 minutes, then add 7.31g (77.7mmol) of 2-aminopyridine and stir at 70°C for 4 hours. TLC monitors that the reaction of 3,4-dibromo-butyric acid ethyl ester is complete, filter, concentrate under reduced pressure, add water , extracted with ethyl acetate (50mL×3 times), combined the organic phases, and removed the solvent under reduced pressure to obtain 10.07g of light yellow oil, which was 2-(imidazo[1,2-α]pyridin-3-yl)acetic acid Ethyl ester, yield 93.2%, purity 97.9%; Among them, the reaction accelerator A is composed of zinc nitrate and glycine with a weight ratio of 5:1, and the mixed solvent is formed by mixing 10ml water and 25ml 1,4-dioxane ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com