Natural product 5,7-dimethoxy-4'-hydroxyisoflavone preparation method
A technology of hydroxy isoflavone and dimethoxy, applied in the direction of organic chemistry, etc., can solve problems such as low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
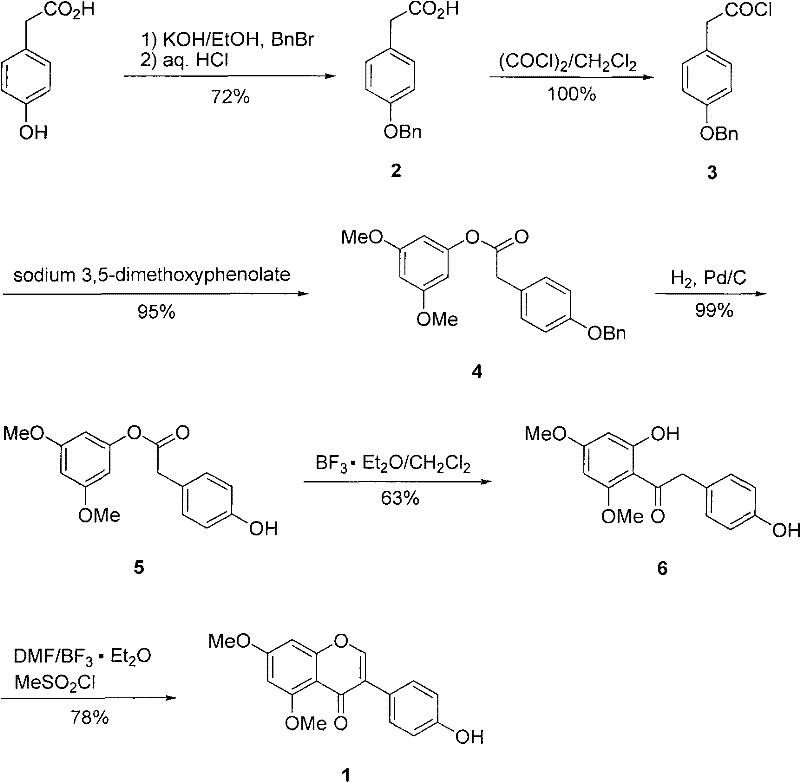
[0025] (1) Synthesis of 2-(4-benzyloxyphenyl)acetic acid (2)
[0026] A water / ethanol (10 / 80mL) solution of potassium hydroxide (4.94g, 88mmol) was added to a suspension composed of p-hydroxyphenylacetic acid (6.08g, 40mmol) and ethanol (20mL), and the reaction mixture was heated at room temperature Stir at low temperature for 5 min, then add benzyl bromide (9.5 mL, 13.68 g, 80 mmol). The reaction mixture was heated to reflux for 2.5 h and cooled to room temperature. Ethanol was distilled off, and 100 mL of 10% sodium hydroxide solution was added to the residue, followed by extraction with ether (30 mL×2). The aqueous phase was acidified with concentrated hydrochloric acid, then extracted with ethyl acetate (30 mL×2), the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain 7.0 g of white crystals of product 2, yield 72%; m.p. 123°C.
[0027] (2) Synthesis of 2-(4-benzyloxyphenyl)acetyl chlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com