Method for preparing 2-bromine-5-fluorobenzotrifluoride
A technology for fluorotrifluorotoluene and m-fluorotrifluorotoluene, which is applied in the field of preparation of chemical intermediates, can solve problems such as difficulty in processing, and achieve the effects of simple post-processing, high reaction yield, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
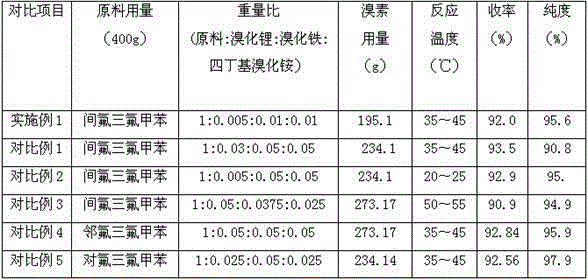
[0023] Example 1: Synthesis of 2-bromo-5-fluorobenzotrifluoride: In a 2000ml three-necked flask, add 400g of concentrated sulfuric acid, 400g of m-fluorobenzotrifluoride, 2g of lithium bromide, 4g of iron bromide, and tetrabutyl bromide successively under stirring in an external water bath Ammonium chloride 4g, bromine 195.1g, controlled temperature 35-45 ℃ for 6h, sampling, GC detection raw material 0.65%. Cool down to below 30°C, filter out the catalyst, transfer to a 2000ml separating funnel, stand still for 1h, separate out the base acid, put the crude product in a 2000ml bottle, add 1000g of water while stirring, drop in 5% sodium hydroxide solution to adjust the pH =7, complete static separation to obtain crude product 550g, yield 92%, content 96%, content 98% through vacuum distillation.
Embodiment 2
[0024] Example 2: In a 2000ml three-necked bottle, add the above batch of separated base acid, then add 400g of m-fluorobenzotrifluoride, 2g of lithium bromide, 4g of iron bromide, 4g of tetrabutylammonium bromide, and 200g of bromine in sequence, and control the temperature at 35-45°C After reacting for 6h, sampling was carried out, and 0.92% of the raw material was detected by GC. Cool down to below 30°C, filter out the catalyst, transfer to a 2000ml separating funnel, stand still for 1h, separate out the base acid, put the crude product in a 2000ml bottle, add 1000g of water while stirring, drop in 5% sodium hydroxide solution to adjust the pH =7, complete static separation to obtain crude product 540g, yield 91.1%, content 95.6%, content 98% through vacuum distillation.
Embodiment 3
[0025] Example 3: In a 2000ml three-necked bottle, add the above batch of separated bottom acid, then add 400g of m-fluorobenzotrifluoride, 2g of lithium bromide, 4g of iron bromide, 4g of tetrabutylammonium bromide, and 235g of bromine in sequence, and control the temperature at 35-45°C After reacting for 6h, sampling was carried out, and 0.92% of the raw material was detected by GC. Cool down to below 30°C, filter out the catalyst, transfer to a 2000ml separating funnel, stand still for 1h, separate out the base acid, put the crude product in a 2000ml bottle, add 1000g of water while stirring, drop in 5% sodium hydroxide solution to adjust the pH =7, complete static separation to obtain crude product 545g, yield 91.9%, content 95.8%, content 98% through vacuum distillation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com