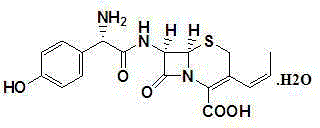
Synthesis method of cefprozil
A technology of cefprozil and synthesis method, which is applied in fermentation and other directions, can solve the problems of many impurities, long reaction cycle of cefprozil, low yield, etc., and achieve the effects of high purity, shortened synthesis cycle, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Take 15g of mother nucleus 7-APRA, add 150ml of purified water and dissolve it with 6mol / L ammonia water, take 13.75g of L-p-hydroxyphenylglycine methyl ester, dissolve it with 6mol / L hydrochloric acid, add it to the mother nucleus 7-APRA solution, add penicillinyl Lyase IPA-750. Stirring was started, and the reaction started for 5 minutes. Before cefprozil was precipitated in the reaction solution, 0.67% (w / v) cefprozil seed crystals (purity greater than 99%) were added at one time. The synthetic pH is 6.5 and the temperature is 15°C.
[0050] After reacting for 2 hours, take samples for HPLC detection. At this time, the residue of mother nucleus 7-APRA is 0.88 mg / ml, and the conversion rate of mother nucleus 7-APRA is 99.1%. Use a sieve to separate penicillin acylase and cefprozil suspension for synthesis, cephalosporin The weight of cefprozil obtained after refining the propylene suspension was 18.54 g, the weight yield was 123.6%, and the purity was 99.51%.
Embodiment 2
[0052] Take 15g of mother nucleus 7-APRA and add 150ml of purified water to dissolve it with 6mol / L ammonia water, then add penicillin acylase PGA-450. Take 13.75g of L-p-hydroxyphenylglycine ethyl ester, dissolve it with 6mol / L hydrochloric acid, and slowly add it dropwise into the mother nucleus 7-APRA solution system. 30 minutes after the dropwise reaction started, before cefprozil was precipitated in the reaction solution, 0.67% (w / v) cefprozil seed crystals (purity greater than 99%) were added in one go. The synthetic pH is 6.5 and the temperature is 15°C.
[0053] After reacting for 2 hours, take samples for HPLC detection. At this time, the residue of mother nucleus 7-APRA is 0.52 mg / ml, and the conversion rate of mother nucleus 7-APRA is 99.5%. Use a screen to separate penicillin acylase and cefprozil suspension for synthesis, cephalosporin The weight of cefprozil obtained after refining the propylene suspension was 18.73 g, the weight yield was 124.9%, and the purity...
Embodiment 3
[0055] Take 15g of the mother nucleus 7-APRA, add 150ml of purified water, dissolve it with 6mol / L ammonia water, and add penicillin acylase for synthesis. Take 13.75g of L-p-hydroxyphenylglycine ethyl ester, dissolve it with 6mol / L hydrochloric acid, and slowly add it dropwise into the mother nucleus 7-APRA solution system. 0-30 minutes after the start of the reaction, 2% (w / v) cefprozil was added in 3 times as a seed crystal (purity greater than 99%). The synthetic pH is 6.0, and the temperature is 20°C.
[0056] After reacting for 2 hours, take samples for HPLC detection. At this time, the residue of mother nucleus 7-APRA is 0.48 mg / ml, and the conversion rate of mother nucleus 7-APRA is 99.5%. Use a sieve to separate penicillin acylase and cefprozil suspension for synthesis, cephalosporin The weight of cefprozil obtained after refining the propylene suspension was 18.70 g, the weight yield was 124.7%, and the purity was 99.58%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com