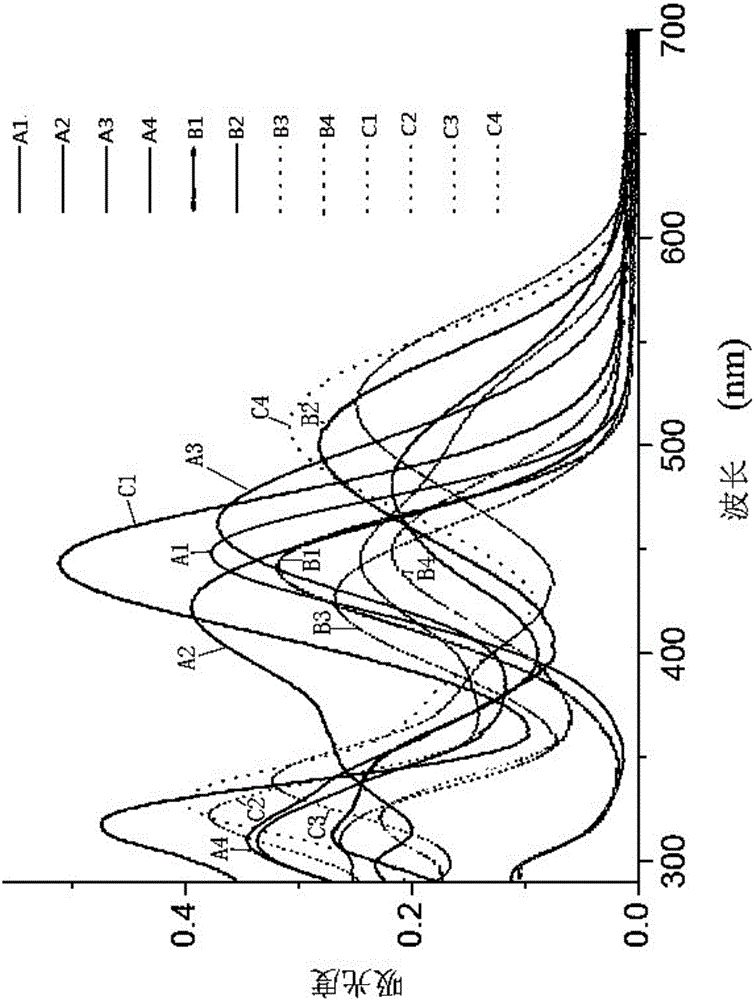
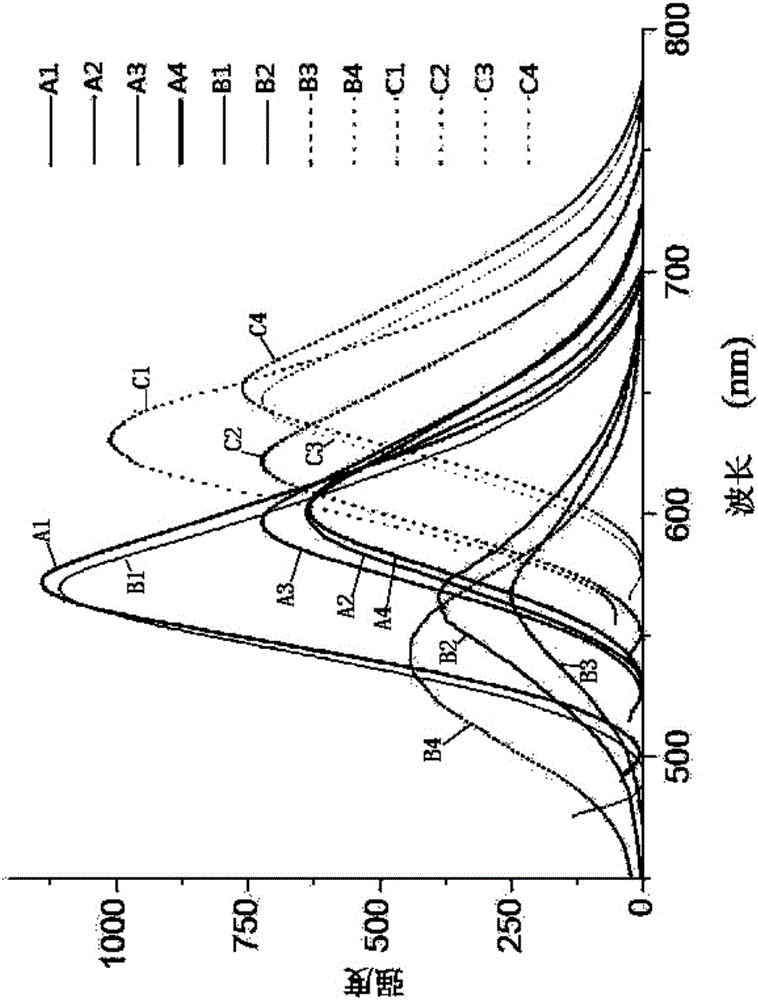
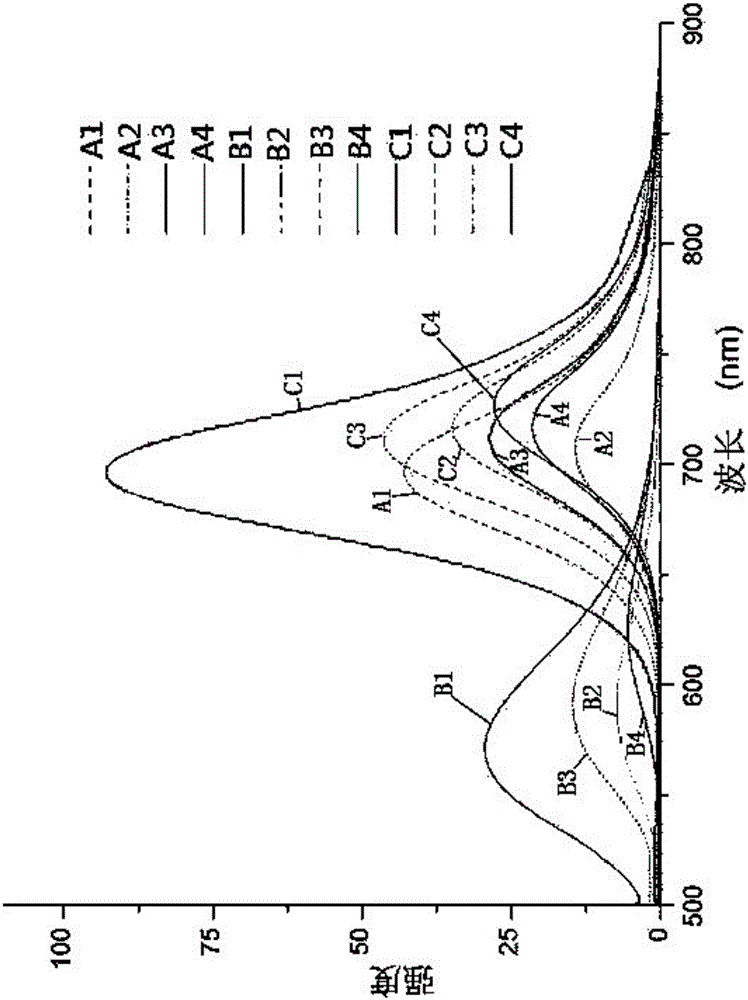
Indole-triphenylamine-arylmethylidenemalononitrile solar energy co-sensitization dye, synthetic method and application thereof
A kind of arylmethylene malononitrile, synthesis method technology, applied in the direction of methine-based/polymethine-based dyes, chemical instruments and methods, organic dyes, etc., can solve the problems of high production cost, high purification difficulty, long synthesis route and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0080] 1. Synthesis of indole-triphenylamine-arylenemalononitrile red light materials: the synthesis route is as follows:
[0081]
[0082] The structural formula of the product is:
[0083]
[0084] 1) Synthesis of compound 2:
[0085]
[0086] Add 4.0g of triphenylamine and 17.8mL of DMF to the single-necked flask, stir to dissolve, slowly add 7.6mL of phosphorus oxychloride in an ice bath, continue stirring for half an hour under ice bath, and after stirring for 1 hour at room temperature, then slowly warm up to 45℃ for reaction 2h. After the reaction, pour the reaction solution into ice water, and neutralize the unreacted POCl with NaOH 3 , Stand and filter to obtain the initial product, recrystallize with ethanol to obtain a pale yellow solid, which is compound 2, the yield is 92%, and the melting point is 132-135°C. 1 HNMR(CDCl 3 , 400MHz, TMS): δ=9.79(s,1H), 7.69-7.71(d,J=8.9,2H), 7.35-7.39(t,J=7.8,4H), 7.18-7.21(m,6H), 7.03 (d, J=8.9, 2H).
[0087] 2) Synthesis of compound 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com