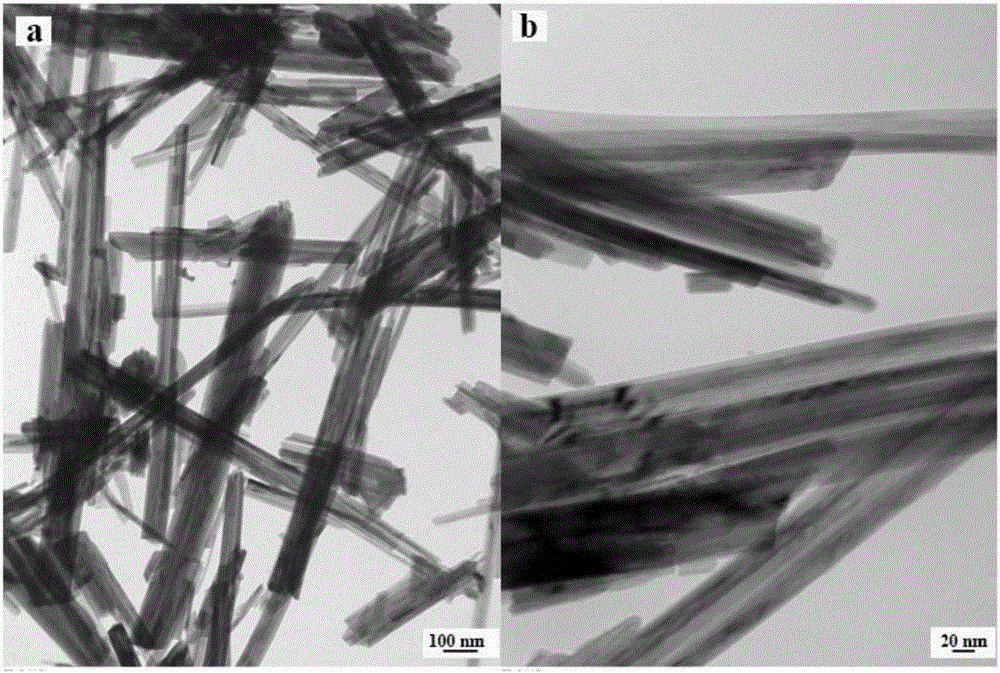
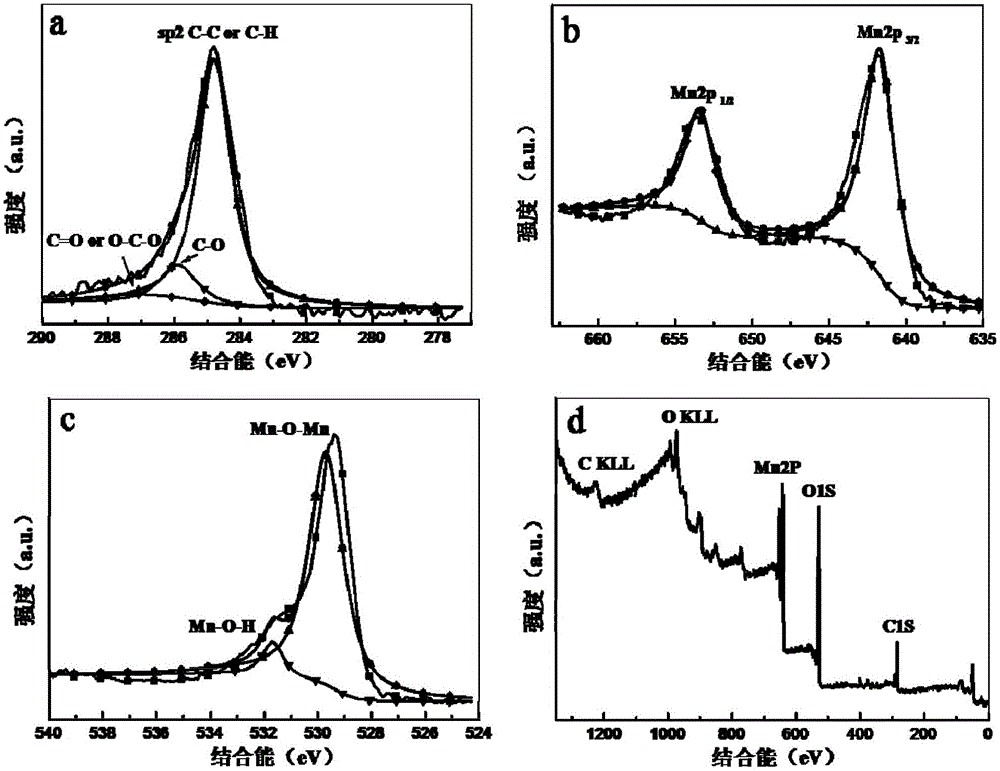
Preparation method and application of graphene quantum dots-MnO2 composite catalyst
A catalyst and graphene-coated technology, applied in nanotechnology for materials and surface science, electrical components, battery electrodes, etc., can solve problems such as poor conductivity and insufficient stability, and achieve low cost and controllable morphology , strong anti-methanol effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: GQDs-1%-MnO 2 -150-6h (GQDs-1% refers to the mass fraction of GQDs in raw materials is 1%, MnO 2 -150-6h refers to MnO 2 The preparation temperature is 150°C, and the preparation time is 6h)
[0038] Step 1: 0.6322g KMnO 4 and 20mL HCl (1mol L -1) into a beaker, added deionized water to prepare a 60mL solution, stirred magnetically for 30min, then transferred to an autoclave and reacted at a constant temperature of 150°C for 6h to obtain a precipitate. The prepared precipitate was washed with deionized water and absolute ethanol, and then vacuum-dried at 80 °C for 8 h to obtain the target product MnO 2 (MnO 2 -150-6h).
[0039] Step 2: Weigh 0.0396g MnO 2 and 0.2mL GQDs solution (2g L -1 ) into a beaker, add deionized water to make a 50mL solution, stir it magnetically for 30min, then transfer it to an autoclave, and react at a constant temperature of 100°C for 24h to obtain a brownish-yellow precipitate. The obtained precipitate was cleaned step by ...
Embodiment 2
[0040] Example 2: GQDs-5%-MnO 2 -150-6h (GQDs-5% refers to the mass fraction of GQDs in raw materials is 5%, MnO 2 -150-6h refers to MnO 2 The preparation temperature is 150°C, and the preparation time is 6h)
[0041] The steps are the same as Step 1 in Example 1.
[0042] Step 2: Weigh 0.0380g MnO 2 In a clean beaker, take 1mL GQDs solution (2g L -1 ) into a beaker, then add deionized water to make a 50mL solution, stir it magnetically for 30min, transfer the uniformly dispersed solution into an autoclave, and react at a constant temperature of 100°C for 24h. The obtained precipitate was cleaned step by step with deionized water and absolute ethanol, dried in a vacuum oven at 80°C for 8 hours, cooled naturally, ground, and weighed to obtain the target product GQDs-MnO 2 (GQDs-5%-MnO 2 -150-6h).
Embodiment 3
[0043] Example 3: GQDs-10%-MnO 2 -150-6h (GQDs-10% means that the mass fraction of GQDs in raw materials is 10%, MnO 2 -150-6h refers to MnO 2 The preparation temperature is 150°C, and the preparation time is 6h)
[0044] The steps are the same as Step 1 in Example 1.
[0045] Step 2: Weigh 0.0540g MnO 2 In a clean beaker, take 3mL GQDs solution (2g L -1 ) into a beaker, then add deionized water to make a 50mL solution, stir it magnetically for 30min, transfer the uniformly dispersed solution into an autoclave, and react at a constant temperature of 100°C for 24h. The obtained precipitate was cleaned step by step with deionized water and absolute ethanol, dried in a vacuum oven at 80°C for 8 hours, cooled naturally, ground, and weighed to obtain the target product GQDs-MnO 2 (GQDs-10%-MnO 2 -150-6h).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com