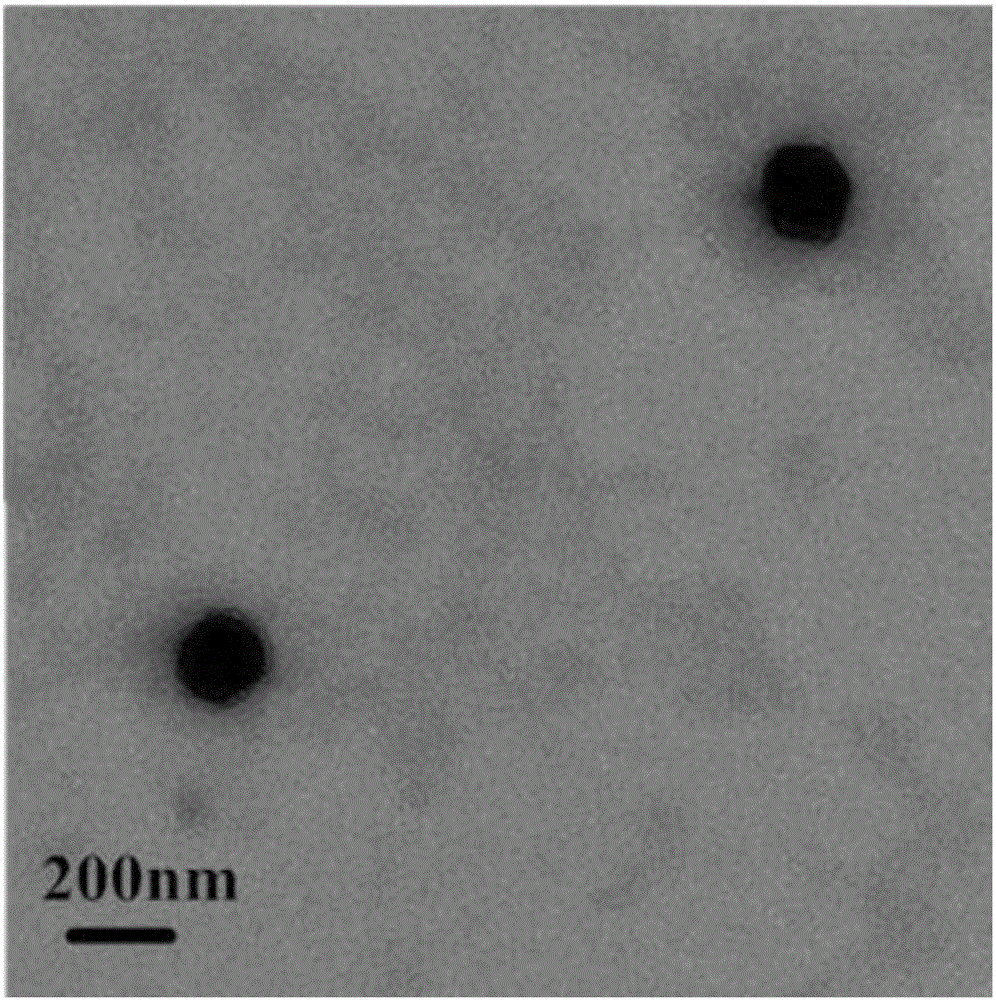
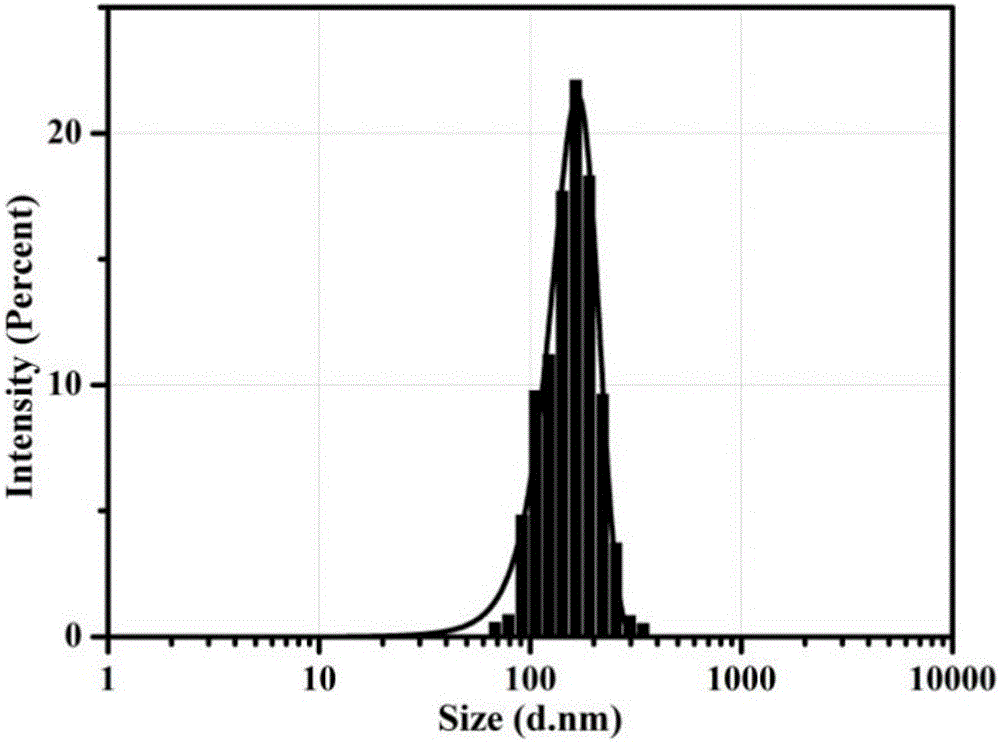
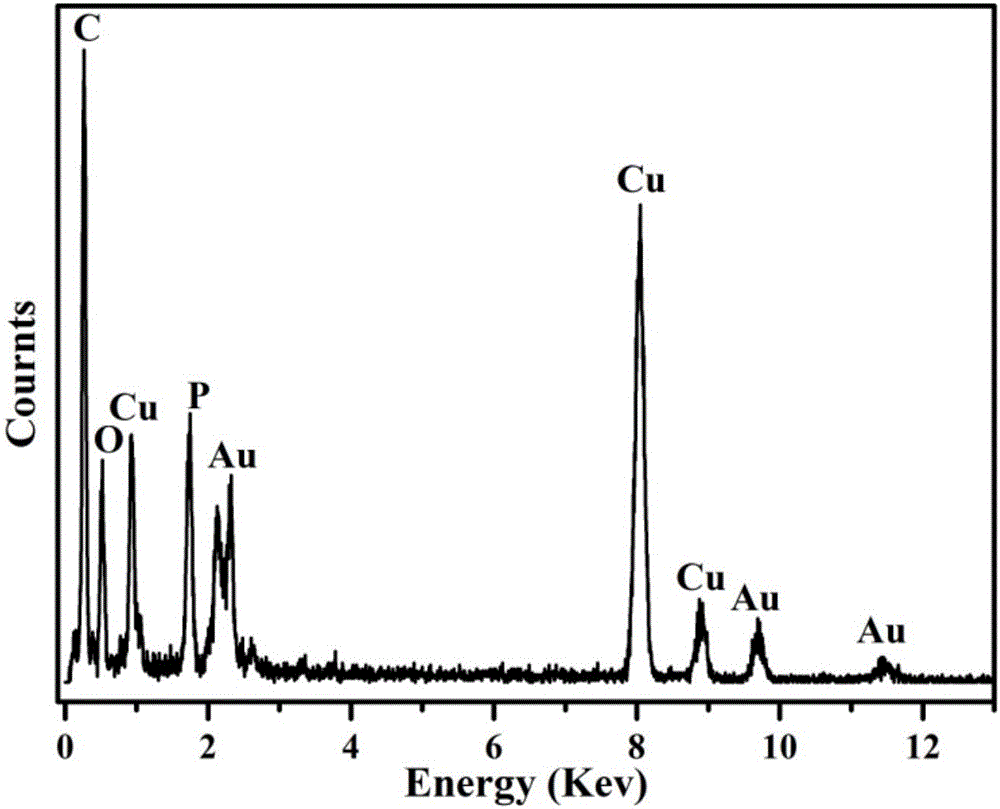
Oleanolic acid liposome coated with nanogold spherical shell and preparation method thereof
A technology of oleanolic acid lipids and nano-liposomes, which is applied in the direction of liposome delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problems of poor targeting, capture, Poor stability and other problems, to achieve the effect of simple preparation method, enhanced stability and high repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 45mg soybean lecithin (purchased from Shenyang Tianfeng Biopharmaceutical Co., Ltd.), 4.0mg cholesterol (purchased from Tianjin Damao Chemical Instrument Supply Station) and 5.0mg oleanolic acid (purchased from Sichuan Shifang Huakang Pharmaceutical Raw Material Factory ), dissolved in 3mL of absolute ethanol, and obtained a homogeneous oil phase, i.e. the organic phase, by magnetic stirring. Under magnetic stirring, 3 mL of the organic phase was slowly and uniformly dropped into 10 mL of PBS buffer solution at 37° C. and pH 6.0 to obtain a liposome suspension. Place on a shaker at 24° C., 100 rpm, and remove absolute ethanol in the liposome suspension for 30 minutes to obtain oleanolic acid nanoliposomes.
[0027] Dissolve 7 mg of chitosan in 1 mL of 0.05 M glacial acetic acid solution to obtain a chitosan solution. Under the stirring of magnetic stirrer, chitosan solution is added dropwise in liposome, and the volume ratio of chitosan and oleanolic acid nano liposome...
Embodiment 2
[0032] 55mg soybean lecithin (purchased from Shenyang Tianfeng Biopharmaceutical Co., Ltd.), 5.5mg cholesterol (purchased from Tianjin Damao Chemical Instrument Supply Station) and 5mg oleanolic acid (purchased from Sichuan Shifang Huakang Pharmaceutical Raw Material Factory) , was dissolved in 5 mL of absolute ethanol, and a uniform oil phase, ie, an organic phase, was obtained by magnetic stirring. Under magnetic stirring, 5 mL of the organic phase was slowly and uniformly dropped into 10 mL of PBS buffer solution at 43° C. and pH 6.8 to obtain a liposome suspension. Place on a shaker at 26° C., 115 rpm, and remove the absolute ethanol in the liposome suspension for 45 minutes to obtain oleanolic acid nanoliposomes.
[0033] Dissolve 10 mg of chitosan in 1 mL of 0.10 M glacial acetic acid solution to obtain a chitosan solution. Under the stirring of magnetic stirrer, chitosan solution is added dropwise in liposome, and the volume ratio of chitosan and oleanolic acid nano li...
Embodiment 3
[0038] 60mg soybean lecithin (purchased from Shenyang Tianfeng Biopharmaceutical Co., Ltd.), 7.0mg cholesterol (purchased from Tianjin Damao Chemical Instrument Supply Station) and 5mg oleanolic acid (purchased from Sichuan Shifang Huakang Pharmaceutical Raw Material Factory) , was dissolved in 6 mL of absolute ethanol, and a uniform oil phase, ie, an organic phase, was obtained by magnetic stirring. Under magnetic stirring, 6 mL of the organic phase was slowly and uniformly dropped into 10 mL of PBS buffer solution at 48° C. and pH 7.4 to obtain a liposome suspension. Place on a shaker at 29° C., 130 rpm, and remove absolute ethanol in the liposome suspension for 60 minutes to obtain oleanolic acid nanoliposomes.
[0039] Dissolve 13 mg of chitosan in 1 mL of 0.15 M glacial acetic acid solution to obtain a chitosan solution. Under the stirring of magnetic stirrer, chitosan solution is added dropwise in liposome, and the volume ratio of chitosan and oleanolic acid nano liposo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com