Substituted phthalazin-1 (2h)-one derivatives as selective inhibitors of poly (adp-ribose) polymerase-1
一种化合物、取代基的技术,应用在制备通式的新化合物领域,能够解决临床益处未获批准等问题
Inactive Publication Date: 2015-09-16
CADILA HEALTHCARE LTD
View PDF29 Cites 19 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0069] Although several compounds have been reported in the literature as PARP-I inhibitors, few have actually demonstrated actual clinical benefit and none have been approved to date
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0111] Example 1: 4-(3-(5-benzyloctahydropyrrolo[3,4-c]pyrrole-2-carbonyl)-4-fluorobenzyl)phthalazin-1(2H)-one;
Embodiment 2
[0112] Example 2: 2-benzyl-5-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoyl)tetrahydropyrrolo[ 3,4-c]pyrrole-1,3(2H,3aH)-dione;
Embodiment 3
[0113] Example 3: 4-(4-fluoro-3-(1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole-5-carbonyl)benzyl)phthalazine-1(2H) -ketone;
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
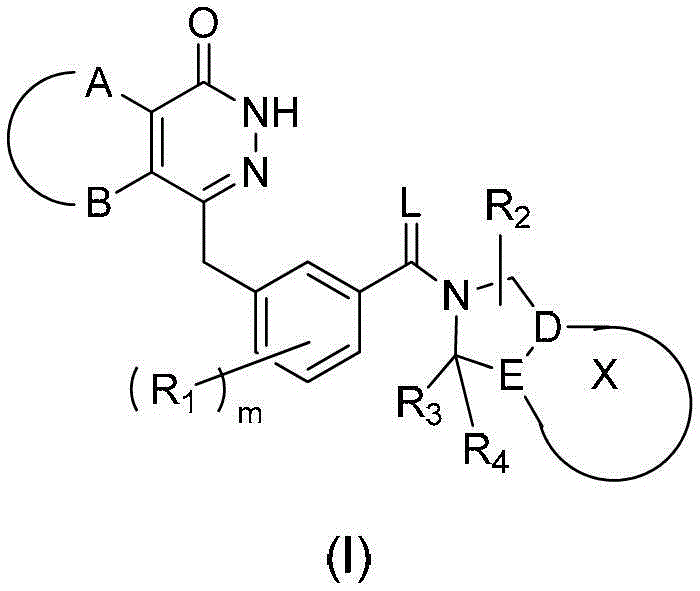
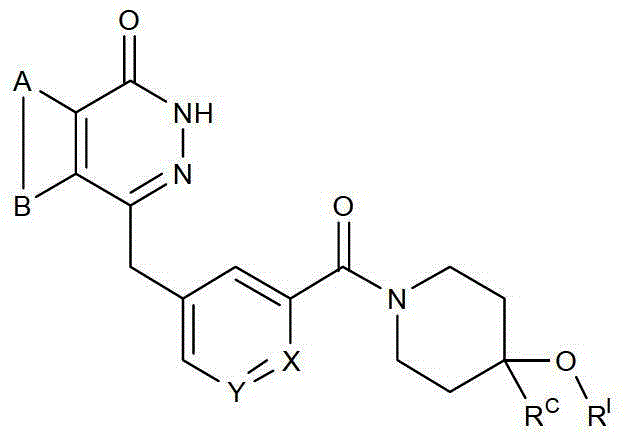
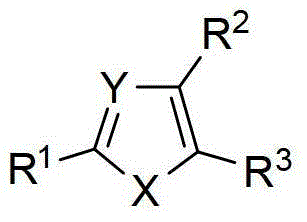
The present invention relates to novel compounds of general formula (I), their stereoisomers, regioisomers, tautomeric forms and novel intermediates involved in their synthesis, their pharmaceutically acceptable salts, pharmaceutically acceptable solvates and pharmaceutical compositions containing them. The present invention also relates to a process of preparing novel compounds of general formula (I), their stereoisomers, regioisomers, their tautomeric forms, their pharmaceutically acceptable salts, pharmaceutically acceptable solvates, pharmaceutical compositions containing them, and novel intermediates involved in their synthesis. The compounds of formula (I) are useful as PARP-1 inhibitors for the treatment of, e.g. cancer.
Description
technical field [0001] The present invention relates to new compounds of general formula (I), their stereoisomers, regioisomers (positional isomers, regioisomers), tautomeric forms and new intermediates involved in their synthesis, and their pharmaceutically acceptable salts , Pharmaceutical solvates and pharmaceutical compositions containing them. The present invention also relates to the preparation of novel compounds of general formula (I), their stereoisomers, regioisomers, their tautomeric forms, their pharmaceutically acceptable salts, pharmaceutically acceptable solvates, pharmaceutical compositions containing them, and Methods of novel intermediates involved in their synthesis. [0002] [0003] The invention further relates to compounds which result in the selective inhibition of poly(ADP-ribose) polymerase-1. Background technique [0004] Exploiting synthetic lethal relationships is a trusted therapeutic strategy to target genetic differences between tumor and...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D237/32C07D487/04C07D491/048C07D495/04C07D498/04C07D513/04A61K31/502A61P35/00
CPCC07D237/32C07D495/04C07D487/04C07D491/048C07D498/04C07D513/04C07D403/10A61P35/00A61P43/00
Inventor 布里杰什·K·斯里瓦斯塔瓦兰吉特·C·德赛潘卡杰·R·帕特尔
Owner CADILA HEALTHCARE LTD
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com