Inhibitors of cxcr1/2 as adjuvants in the transplant of pancreatic islets
A technology of islet transplantation and inhibitors, applied in medical preparations containing active ingredients, active ingredients of anhydride/acid/halide, allergic diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
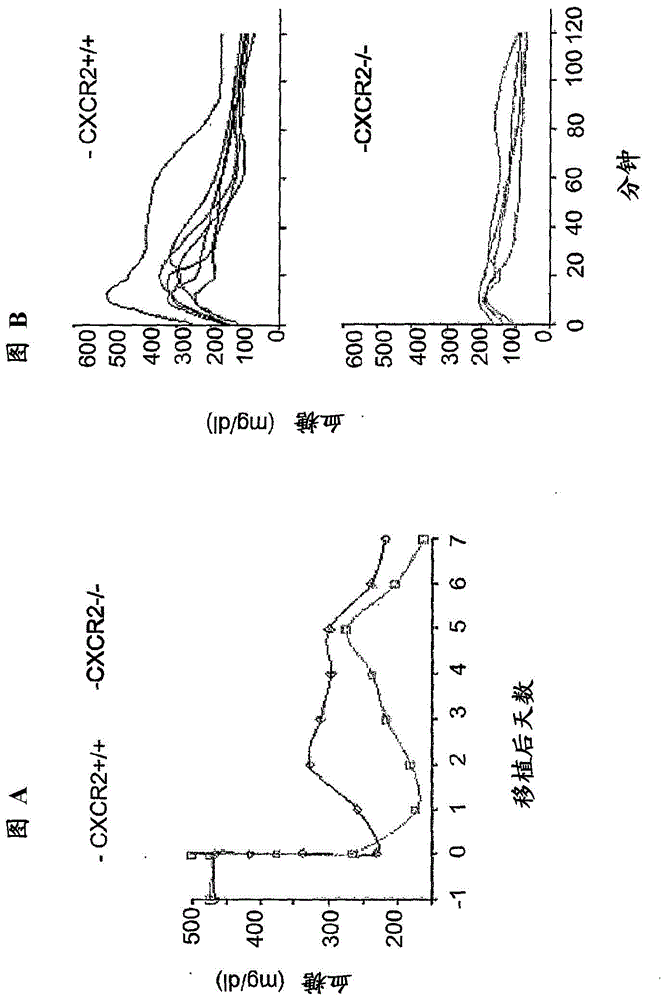
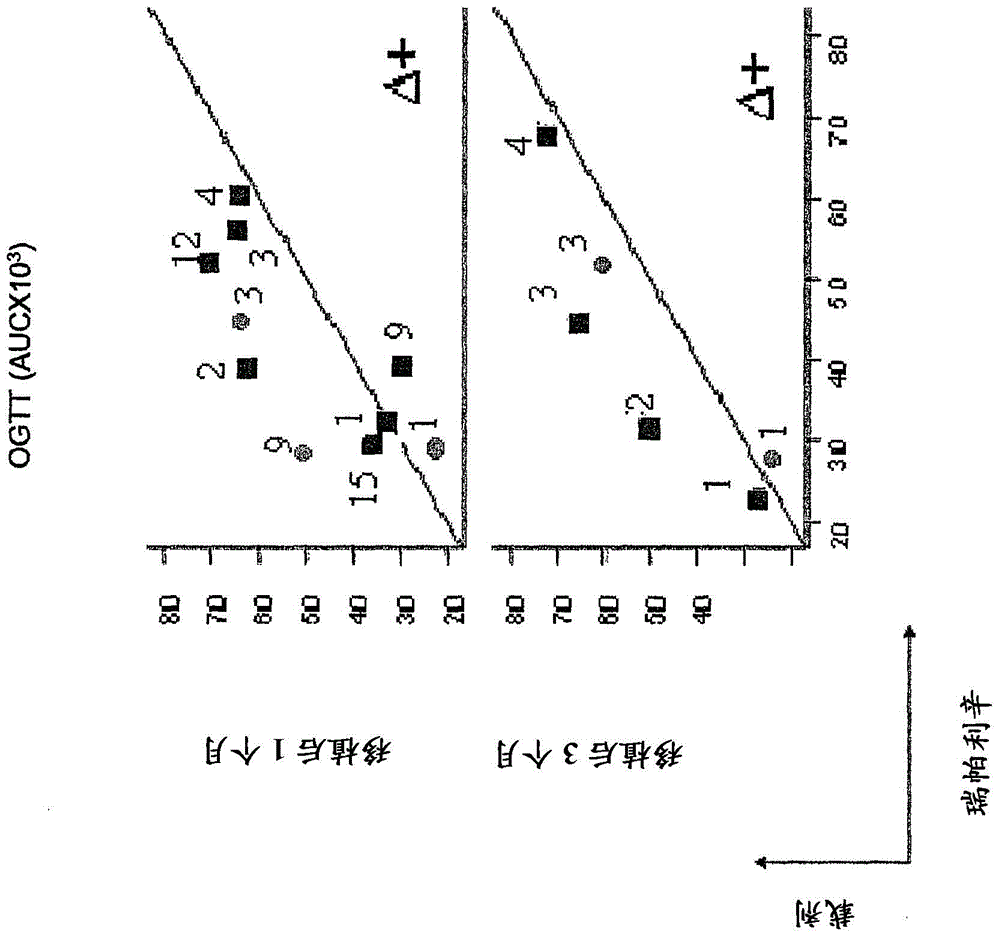
[0047] As will be described below, the compound of formula I is effective in improving the survival and function of the implant after islet transplantation in animal models.
[0048] In detail, the data obtained in the experimental model of islet transplantation demonstrated that the above compounds (represented by R(-)-2-[(4-isobutylphenyl)propionyl]-methanesulfonamide) were β-cells from loss of activity and / or degeneration of the apparent role.
[0049] According to a preferred embodiment of the present invention, the compound of formula I is Reparixin. According to another preferred embodiment, the compound of formula I is maricin.
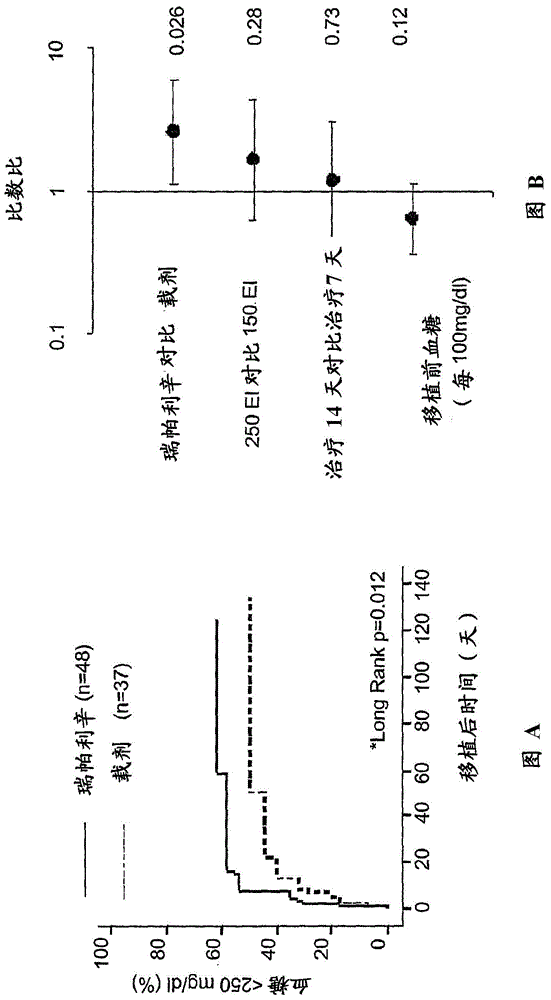
[0050] Compounds of the invention effectively support engraftment of transplanted islet cells in type 1 diabetes.
[0051] In fact, as will become clearer from the experimental section below, intravenous administration of Reparixin in animal models from -1 to +6 days of syngeneic or allogeneic islet transplantation elicited up to Median ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com