Anti-MIF antibody cell migration assay
A measurement method, a technology of cell migration, applied in chemical instruments and methods, specific peptides, measuring devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0112] The preparation of the anti-(ox)MIF antibody of the present invention includes any method using genetic engineering to produce recombinant DNA, for example, by reverse transcription of RNA and / or amplification of DNA and cloning it into an expression vector. In some embodiments, the vector is a viral vector, wherein additional DNA segments can be ligated into the viral genome. In some embodiments, the vector is capable of autonomous replication in the host cell into which it is introduced (eg, bacterial vectors and episomal mammalian vectors with a bacterial origin of replication). In other embodiments, the vector (eg, a non-episomal mammalian vector) can integrate into the genome of the host cell when introduced into the host cell, thereby replicating along with the host genome. Furthermore, certain vectors are capable of directing the expression of genes to which they are operably linked. Such vectors are referred to herein as "recombinant expression vectors" (or sim...
Embodiment 1
[0170] Anti-MIF Antibody RAM9 Chemical Activation Assay; Cell-Based Assay
[0171] Intended purpose:
[0172] The assay was set up to test the function (= test item) of glycine buffered anti-MIF RAM9 preparations to inhibit the random migration (= chemical activation) of monocytes. The inventors have demonstrated that this assay, as well as the respective methods, can be used as a quality control test in the processing steps of the final drug product (FDP).
[0173] 1) Principle:
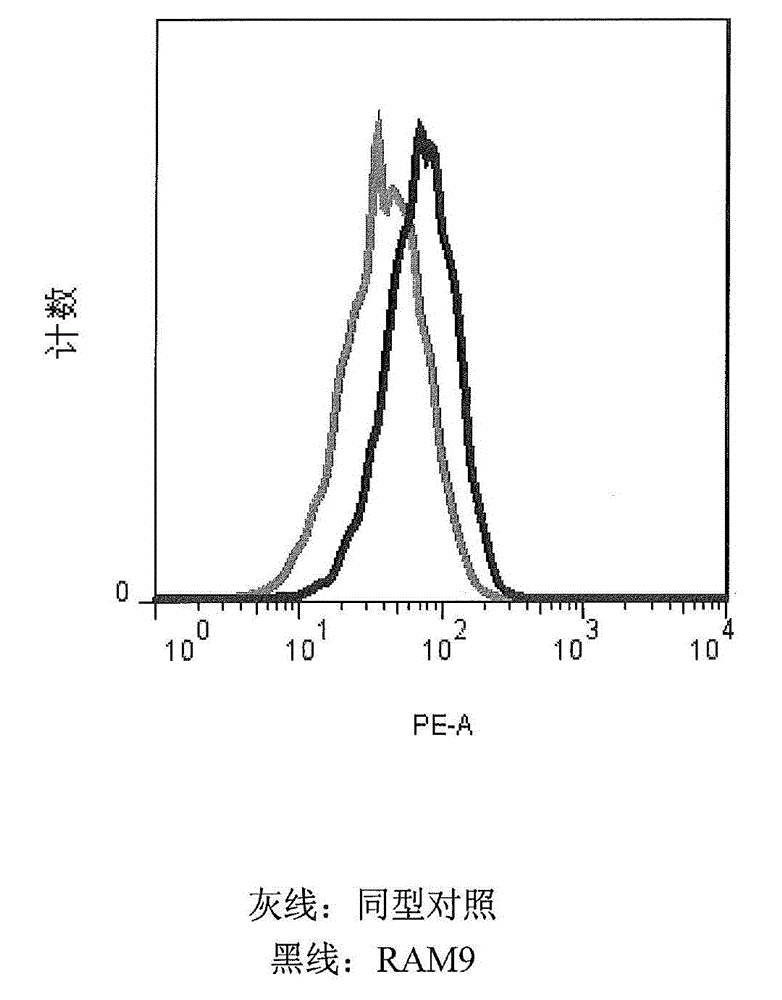
[0174] MIF is constitutively expressed on U397 (and other cancerous) monocytes and oxMIF is present on the surface of these cells (FACS data, see figure 2 ), which supports the migration function. It is an important feature of the invention to use cells expressing (ox)MIF endogenously or exogenously, eg cells from disease samples. This rationale is based on the inventors' previous findings that oxMIF is absent on healthy cells or tissues. The U397 cell line is a preferred embodiment for carryi...
Embodiment 2
[0242] In principle, this same assay can be used to determine whether antibodies can be provided to the upper chamber together with the cells.
[0243] summary:
[0244] Cell migration test: HTS Transwell plate (Coming): 5μm
[0245] *Cells: U937 (passage 10), starved overnight
[0246] *RAM9 Antibody: 30nM-0.04nM, pipet into culture chamber
[0247] * Glycine: 30nM; 10nM; 3,3nM Pipette into the culture chamber
[0248] *Negative Control: Glycine; Positive Control: FBS (Fetal Bovine Serum)
[0249] *Culture: 2 plates, 16 hours
[0250] * calculations for the results
[0251] result:
[0252] Tablet 1
[0253] Antibody concentration (nM)
Measurements
fit
0
1997,6
0,04
2452,7
2498,3
0,12
2490,8
2388,4
0,37
2019,2
2106,0
1,1
1642,0
1621,2
3,3
1179,6
1115,5
10
688,0
810,8
30
753,7
685,7
maximum value
=A
2555
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com